Nanoparticles are nanosized clusters with dimensions less than 100nm. Nanoparticles are fabricated by physical, chemical, and biological methods. Physical and chemical methods are energy intensive and involve hazards of contaminations. Biological synthesis of nanoparticles is environment friendly, less toxic and cost effective process. Plants, microorganisms, and biomolecules are commonly exploited species for merging of nanoparticles in this method. In present work we synthesize Chromium oxide nanoparticles by biological method using fungal extract of Aspargillus Niger. The synthesized nanoparticles are characterized by XRD (X-Ray Diffraction), SEM (Scanning Electron Microscopy) and UV-Vis (Ultraviolet Visible) techniques.
Nanotechnology is an escalating field in recent science [1] that controls matter which is extremely small (< 100nm). Idea of Nanotechnology was proposed by Richard Feynman [2] and Professor Norio Taniguchi coined this term in 1974. Nanotechnology is the segregation, organization, and strengthening of materials. Nanoparticles are ultrafine particles [3] with exceptional physico-chemical characteristics which are the result of quantum confinement effect [4]. In particles of the size less than 70nm Vander waals force becomes significant. Gecko can climb the walls due to nanosized hairs on its limbs. Gold NPs change their ability to reflect light and Al NPs are extremely reactive when their size is less than 20nm. Nanoparticles are fascinating because of their change of properties when they are very small [5]. Concept of Nanoparticles is not new, they have been in use since 4th century A.D, for example Lycurgus cup [6]. Nanoparticles have various applications in catalysis, electronic devices, dyes and pigments [6, 7].
Physical, chemical, and biological methods are commonly used for the synthesis of nanoparticles. Physical methods are free from contaminants but are uneconomical because of formation of plentiful waste. Physical methods used for the synthesis of nanoparticles are ball-milling, ablation, and pyrolysis. Various chemical methods used for the synthesis of nanoparticles are sol-gel method, microemulsion, hydrothermal, and chemical vapor deposition. Biological methods are clean and environment friendly methods. Various biological entities like fungi, bacteria, and plants are used in this method. This method involves enzymes, proteins, and NADH reductase coenzyme [8].
Biological methods using microorganisms are clean, nonhazardous, and eco friendly. These are fast and are carried out at ordinary conditions. Microorganisms are adapted to harsh conditions by using survival strategies such as efflux system, extracellular precipitation and chemical detoxification. Reduction, biosorption, and bioaccumulation are important mechanisms for biosynthesis [9, 10].
Chromium (iii) oxide nanoparticles are one of the unique transition metal compounds [11] that have won much attention of researchers because of their extensive use in science and technology [12]. The \(Cr_2O_3\) nanoparticles are manufactured by an aqueous precipitation method using chromic sulphate as a template and ammonia as a precipitating agent. Gibot and Vidal synthesized spherical \(Cr_2O_3\) nanoparticles by thermal decomposition of \(Cr (NO_3)_3.9H_2O\) [13]. Pei et al. synthesized \(Cr_2O_3\) nanoparticles by using \(CrO_3\) and \(C_2H_5OH.\) Jaswal et al. synthesized \(Cr_2O_3\) NPs by precipitation method using chromic sulphate and ammonia. This method is of low cost and environment friendly [14].
\(Cr_2O_3\) NPs are fabricated by reducing potassium dichromate solution with Tridaxprocumbens leaf extract [15] and Allium sativum [16]. Pure and uniform sized \(Cr_2O_3\) NPs with cubic morphology were synthesized by a green chemistry method using Callistemon viminalis flowers extract [17]. Chromium oxide NPs were synthesized by mixing potassium dichromate solution with pumpkin leaves extract. The solution was dried at 70°C for 6 hours and then calcined at 650°C [18]. The use of bioorganisms is ecofriendly approach with natural reducing and capping agents [19]. Chromium oxide NPs find wide range of applications in colorants, catalysts, coatings [20], green pigment, solar energy collectors, and liquid crystal displays [21].
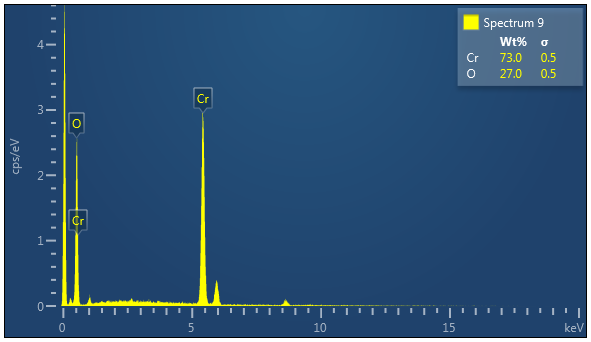
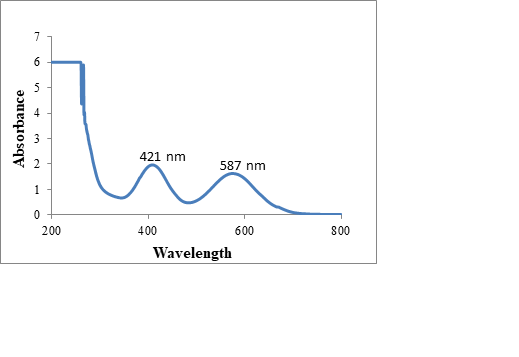
We analyzed nanoparticles by using XRD model D8 ADVANCE BRUKER X-Source Copper/(anode). UV-Vis is performed on UV-Vis Spectrometer Perkin Elmer, Lambda 25. Both instruments are placed at Nanoscience and Technology Department, Quaid-e-Azam University, Islamabad. The Scherrer formula is used for finding size. The formed NPs are characterized by XRD and their results arenoted in nanometer. The synthesized NPs are also characterized by SEM performed on SEM, TESCAN, VEGA3 placed at Advanced Energy and Material lab NUST. The SEM study is carried out to find size and morphology of nanoparticles. The EDX is done on EDX Oxford placed at Fracture Mechanics and Fatigue Lab, Mechanical Engineering Department, UET Taxila. The EDX is used to find elemental composition and purity of samples.
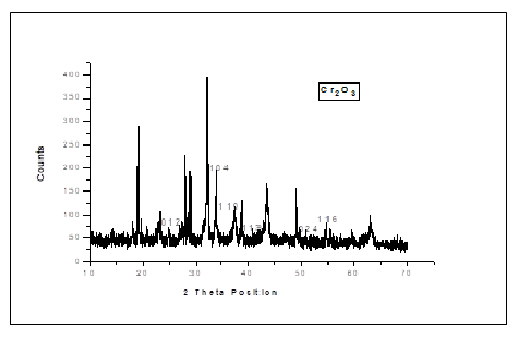
| PEAKS | 2θ POSITION | hkl VALUES | d-SPACING |
|---|---|---|---|
| 1 | 24.720 | 012 | 3.5986 |
| 2 | 33.912 | 104 | 2.6412 |
| 3 | 36.530 | 110 | 2.4578 |
| 4 | 41.865 | 113 | 2.1560 |
| 5 | 50.696 | 024 | 1.7992 |
| 6 | 55.381 | 116 | 1.6576 |
| 7 | 65.760 | 300 | 1.4189 |