Open Journal of Chemistry
ISSN: 2618-0758 (Online) 2618-074X (Print)
DOI: 10.30538/psrp-ojc2018.0003
Different Approaches for the Synthesis of Zinc Oxide Nanoparticles
Zaheer Ahmad, Farman UllahKha, Sajid Mahmood\(^1\), Tariq Mahmood, Aisha Shamim
Department of Chemistry, University of Wah, Wah Cantt, 47040 –Pakistan.; (Z.A & F.U.K & A.S)
Department of Chemistry, Division of Science & Technology,University of Education, Township Campus, Lahore, Pakistan.; (S.M)
Nano Science & Technology Department, National Centre for Physics, Quaid-e-Azam University, Islamabad 45320-Pakistan.; (T.M)
\(^{1}\)Corresponding Author; drsajidue@gmail.com
Abstract
Keywords:
1. Introduction
The nanotechnology is an area of intense scientific research in which nanomaterials are synthesized, characterized and applied. Nanoparticles are the clusters of atoms, ions or molecules who's size range is < 100nm. These include fullerenes, metal clusters, proteins etc. Many nanomaterial are formed by metals like Cobalt (Co), Copper (Cu), Zinc (Zn), Magnesium (Mg), Titanium (Ti), Gold (Au) and Silver (Ag). Metal oxides nanoparticles can be prepared easily.Due to their small size they differ from the bulk materials. The nanoparticles are being used for different purposes i.e. for medical treatments, in different branches of industry productions such as solar and oxide fuel batteries for energy storage, for incorporation into various materials of daily use e.g. cosmetics or clothes. The Zinc oxide (ZnO) nanoparticles has been widely used in the light emitting diodes, solar cells, piezoelectric transducers, gas sensors and as catalysts for long time. ZnO is one of the n-type semiconductor which have bandgap of about 3.37ev and has been used in different fields of catalysis, sensors, electronic devices, solar cells. As the ZnO nanoparticles have smaller size so they have large surface area.They have been synthesized by different methods so far. In 2010, they were synthesized from aqueous solutions in the form of equiaxed nanoparticles [1].They show some photocatalytic properties and are widely used in sunscreen cosmetics, clothes and also for the degradation of environmental pollutants. They have some biological activities also. In 2012, the antitumor activity of the photostimulated ZnO nanoparticles was studied. They were used with cisplatin and the results showed the increased antitumor activity [2]. In 2013, ZnO nanoparticles were also synthesized by using supercritical methanol in good yield [3]. They can be synthesized with combination of transition metals e.g. through the electrolysis process in which Zinc plate is used as anode in sodium tungstate solution [4].They are very effective against UV blocking and for in vivo toxicity of the polymer coated. The ZnO nanoparticles coated with chitosan (ZnO-CTS) and polyethylene glycol (PEG) which is a synthetic polymer (ZnO-PEG) have also been synthesized and their photocatalytic activity was studied which showed the increased photocatalytic activity and stability and which also showed the better ultraviolet absorption efficiency [5].They can also be produced through hydrothermal method in which microwave is used. In this process by changing in the power and the time for microwave irradiation cause different morphological effects on the ZnO nanoparticles [6].The ZnO nanoparticles were observed to cause eosinophilc airway inflammation in mice [7].2. Method and materials
2.1. Materials
Zinc Chloride (ZnCl2), Sodium Hydroxide (NaOH). All these chemicals were of AR grade and purchased from Sigma Aldrich.2.2. Procedure
We took 5g of Zinc Chloride (ZnCl2) and dissolved in small amount of distilled water to make its solution. This Solution was titrated with 0.5M Sodium Hydroxide (NaOH) solution unless white precipitate appeared. Water was added in this precipitate and then washed with distilled water 5 times. This precipitate was filtered. The precipitate was separated and then put it in hot air oven at a temperature of 1050C for 3 hours. Then it was grind with mortar and pestle for 15 minutes and then put it in muffle furnace at 300°C for 5 hours for calcination. After calcination its color changed to cream yellow from white. Then was grind again and stored in glass bottle for further process.3. Biological synthesis of Zno nanoparticles
3.1. Materials
Zinc chloride (ZnCl2), Sodium hydroxide (NaOH), Deionized water, Aspargillus niger. All the chemicals were of AR grade and purchased from Sigma Aldrich.3.2. Procedure
In this method first of all salt solution was prepared by dissolving 5g of salt in deionized water and mixed with 2 g crushed powder of Aspargillus niger and stirred for 30 minutes. Then the resultant material was placed in dark for 3 days. After 3 days the solution was filtered. The pale white filtrate was obtained. This filtrate was dried in hot air oven at105°C and then calcined in muffle furnace at 550°C for 3 hours .The material was grind and stored for further analysis.4. Results and discussion
For the characterization of prepared Zinc Oxide nanoparticles, XRD is one of the best technique. It characterizes the purity and phase of the nanomaterial. XRD gives detail of diffraction angle, the interlayer spacing and mainly the crystallite size. The XRD used in our work was Bruker D8 advance.Figure.1 shows the XRD pattern of zinc oxide through chemical method. The peaks at \(2\theta=32.0\), 34.5, 36.0, 47.5, 56.0, 63.0, 67.0, 68.0 and 69.0 which correspond to (100), (002), (101), (102), (110), (103), (200), (112) and (201) crystalline planes of ZnO and were in good agreement with the JCPDS Card no. 01-079-2205. All these peaks exactly match with the literature [8, 9] which clearly indicates the formation of zinc oxide. The average crystallite size of ZnO NP's was 71.5 nm, calculated using Scherrer equation based on the full width at half-maximum of the (101) diffraction planeFigure 1. XRD pattern of Zinc Oxide NP (chemical method)
Figure 2. XRD pattern of Zinc Oxide NP (biological method)
Figure 3. SEM images of ZnO NP (chemical method)
Figure 4. SEM images of ZnO NP (biological method)
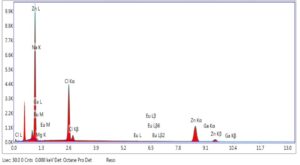
Figure 5. EDX spectra of ZnO NP (chemical method)
5. Conclusion
In summary, The Zinc Oxide nanoparticles were successfully synthesized by two different methods i.e. chemical and biological. For biological synthesis Aspargillus niger was used. The product obtained was characterized through different analytical techniques like XRD, SEM and EDX.The obtained results weretallied with literature. It was revealed that the ZnO nanoparticles can be prepared from Aspargillus niger in sufficient quantity with high purity.Acknowledgement
This work was impossible without the help and guidelines of my supervisor Dr. Zaheer Ahmad, Chemistry Department University of Wah, Wah Cantt and co-supervisor Dr. Tariq Mahmood, NCP, Islamabad. I cordially thank them for their support and guidance throughout this research work.Competing Interests
The author do not have any competing interests in the manuscript.References
- Lu, K., & Zhao, J. (2010). Equiaxed zinc oxide nanoparticle synthesis. Chemical Engineering Journal, 160(2), 788-793. [Google Scholor]
- Hackenberg, S., Scherzed, A., Harnisch, W., Froelich, K., Ginzkey, C., Koehler, C., ... & Kleinsasser, N. (2012). Antitumor activity of photo-stimulated zinc oxide nanoparticles combined with paclitaxel or cisplatin in HNSCC cell lines. Journal of Photochemistry and Photobiology B: Biology, 114, 87-93. [Google Scholor]
- Kim, M., Lee, H. S., Yoo, S. J., Youn, Y. S., Shin, Y. H., & Lee, Y. W. (2013). Simultaneous synthesis of biodiesel and zinc oxide nanoparticles using supercritical methanol. Fuel, 109, 279-284.[Google Scholor]
- Rahimi-Nasrabadi, M., Pourmortazavi, S. M., Ganjali, M. R., Hajimirsadeghi, S. S., & Zahedi, M. M. (2013). Electrosynthesis and characterization of zinc tungstate nanoparticles. Journal of Molecular Structure, 1047, 31-36. [Google Scholor]
- Girigoswami, K., Viswanathan, M., Murugesan, R., & Girigoswami, A. (2015). Studies on polymer-coated zinc oxide nanoparticles: UV-blocking efficacy and in vivo toxicity. Materials Science and Engineering: C, 56, 501-510. [Google Scholor]
- Hasanpoor, M., Aliofkhazraei, M., & Delavari, H. (2015). Microwave-assisted synthesis of zinc oxide nanoparticles. Procedia Materials Science, 11, 320-325.[Google Scholor]
- Huang, K. L., Lee, Y. H., Chen, H. I., Liao, H. S., Chiang, B. L., & Cheng, T. J. (2015). Zinc oxide nanoparticles induce eosinophilic airway inflammation in mice. Journal of hazardous materials, 297, 304-312.[Google Scholor]
- Akhtar, M. J., Ahamed, M., Kumar, S., Khan, M. M., Ahmad, J., & Alrokayan, S. A. (2012). Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. International journal of nanomedicine, 7, 845. [Google Scholor]
- Hosseini, S. A., & Babaei, S. (2017). Graphene oxide/zinc oxide (GO/ZnO) nanocomposite as a superior photocatalyst for degradation of methylene blue (MB)-process modeling by response surface methodology (RSM). Journal of the Brazilian Chemical Society, 28(2), 299-307. [Google Scholor]
- Mohan, A. C., & Renjanadevi, B. (2016). Preparation of zinc oxide nanoparticles and its characterization using scanning electron microscopy (SEM) and X-ray diffraction (XRD). Procedia Technology, 24, 761-766. [Google Scholor]
- Geetha, A., Sakthivel, R., Mallika, J., Kannusamy, R., & Rajendran, R. (2016). Green Synthesis of antibacterial Zinc oxide Nanoparticles using biopolymer Azadirachtaindica gum. Oriental Journal of Chemistry, 32(2), 955-963. [Google Scholor]