Open Journal of Chemistry
ISSN: 2618-0758 (Online) 2618-074X (Print)
DOI: 10.30538/psrp-ojc2019.0011
The primary corrosiveness of crude oils and destructions of metals
Suresh Aluvihara\(^1\), Jagath K. Premachandra
Department of Chemical and Process Engineering, University of Peradeniya, Peradeniya, Sri Lanka.; (S.A)
Department of Chemical and Process Engineering, University of Moratuwa, Katubedda, Sri Lanka.; (J.K.P)
\(^{1}\)Corresponding Author; sureshaluvihare@gmail.com
Abstract
Keywords:
1. Introduction
Corrosion is a common term germane the industry of crude oil refining similarly generate a few of problems on such ferrous metals. Upon the hole the corrosion is the formation of metal oxides, sulfides, hydroxides or other certain compound on the surface of such a metal yet the rate of corrosion is depend on the conditions of the surrounding and chemical composition of such metal as well [1]. As an essential requirement of the corrosion the metal need to be exposed either some kind of stronger oxidizing agent than \(Fe_2+\) or any environment consisting water and oxygen also the process will be modified by the acids and salt present in the medium. Beside of that the mercaptans and elemental sulfur play a dominant role in the cause of metallic corrosion [2]. Those compounds are found in crude oil in various amounts. Therefore, the crude oil can be emphasized as a corrosive admixture especially in the industry of crude oil refining because of the vast applications of ferrous metals with regard to different units such as the distillation column, storage tanks and transportation tubes. In the current research there were expected to investigate the effect of different two types of crude oils on the rates of corrosion in seven different types of ferrous metals and the variations of the initial hardness of such metals due to the corrosion.2. Materials and methodology
2.1. Materials
2.1.1. Ferrous metals
Seven different types of ferrous metals were selected as the samples which are having foremost applications in the industry of crude oil refining frequently. A batch of samples was consisted with three different types of carbon steels, three different types of stainless steel and Monel.2.1.2.Crude oils
It was selected two different types of crude oils which were used in the Sri Lankan crude oil refining industry and also those are slightly different in their chemical composition including essential properties such as corrosive properties.
2.2. Methods
2.2.1.Experiments on crude oils
The sulfur content, Mercaptans content, acidity and the salt content of both crude oils were determined. Because those are the dominant corrosive properties found in crude oils. A descriptive summary of the relevant procedures of experiments for such properties has been given in the Table 1.
Table 1. Procedures of the experiments for the corrosive properties in crude oils.
{Property}
{Method}
{Readings}
Sulfur content
Directly used
Direct reading
Acidity
Each sample was dissolved in a mixture of
toluene and isopropyl and titrated with
potassium hydroxide.
End point
Mercaptans content
Each sample was dissolved in sodium acetate
and titrated with silver nitrate.
End point
Salt Content
Each sample was dissolved in organic solvent
and exposed to the cell analyzer.
sample
There were determined the ferrous concentration of crude oil samples which were exposed to the carbon steels and stainless steels by the atomic absorption spectroscopy (AAS). It was also tested the copper concentrations in crude oil samples which were exposed to Monel by the same instrument because of the confirmation evidences for the corrosion eventually.
2.2.2.Experiments on metals
The elemental composition of each type of metal was determined by the XRF detector due to the importance of the chemical composition on the rate of corrosion. According to the working principles of the XRF detector it was able to detect the compositions of each metal and even though most of nonmetals without carbon.
A batch of similar sized metal coupons was prepared from seven different types of ferrous metals. Those metal coupons were immersed homogeneously in separate crude oil samples as shown in the Figure 1.
Table 1. Procedures of the experiments for the corrosive properties in crude oils.
| {Property} | {Method} | {Readings} |
|---|---|---|
| Sulfur content | Directly used | Direct reading |
| Acidity | Each sample was dissolved in a mixture of toluene and isopropyl and titrated with potassium hydroxide. |
End point |
| Mercaptans content | Each sample was dissolved in sodium acetate and titrated with silver nitrate. |
End point |
| Salt Content | Each sample was dissolved in organic solvent and exposed to the cell analyzer. |
sample |
2.2.2.Experiments on metals The elemental composition of each type of metal was determined by the XRF detector due to the importance of the chemical composition on the rate of corrosion. According to the working principles of the XRF detector it was able to detect the compositions of each metal and even though most of nonmetals without carbon. A batch of similar sized metal coupons was prepared from seven different types of ferrous metals. Those metal coupons were immersed homogeneously in separate crude oil samples as shown in the Figure 1.
Figure 1. (a) Samples (b) Set up of apparatus
3. Results and Discussion
3.1. Chemical Compositions of Metals
According to the readings of XRF detector the elemental compositions of ferrous metals are given in the Table \ref{t2}.Table 2. Chemical compositions of metals.
| Metal | Fe (\%) |
Mn (\%) |
Co (\%) |
Ni (\%) |
Cr (\%) |
Cn (\%) |
P (\%) |
Mo (\%) |
Si (\%) |
S (\%) |
Ti (\%) |
V (\%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbon Steel (High) | 98.60 | 0.43 | - | 0.17 | 0.14 | 0.7 | 0.12 | 0.086 | 0.09 | - | - | - |
| Carbon Steel (Medium) | 99.36 | 0.39 | - | - | - | - | 0.109 | - | 0.14 | <0.002 | <0.04 | - |
| Carbon Steel (Mild Steel) | 99.46 | 0.54 | <0.30 | - | <0.07 | - | - | - | - | - | <0.19 | <0.07 |
| 410-MN: 1.8 420-MN: 2.8 (Stainless Steel) |
88.25 | 0.28 | - | 0.18 | 10.92 | 0.10 | 0.16 | - | 0.11 | - | - | - |
| 410-MN: 1.7 420-MN: 1.7 (Stainless Steel) |
87.44 | 0.30 | - | - | 11.99 | - | 0.18 | - | 0.09 | - | - | - |
| 321-MN: 1.4 304-MN: 1.9 (Stainless Steel) |
72.47 | 1.44 | - | 8.65 | 17.14 | - | 0.18 | - | 0.12 | - | - | - |
| Moneal 400 | 1.40 | 0.84 | 0.11 | 64.36 | <0.04 | 33.29 | - | - | - | - | - | - |
3.2. Corrosive Properties of Crude Oils
According to the quantitative analysis of the corrosive properties the numerical values for those properties of both crude oils are given in the Table \ref{t3}.Table 3. Corrosive properties of crude oils.
| Property | Murban | Das Blend |
|---|---|---|
| Sulfur Content (Wt. W%) | 0.758 | 1.135 |
| Salt content (ptb) | 4.4 | 6.3 |
| Acidity (mg KOH/g) | 0.01 | 0.02 |
| Mercaptans content (ppm) | 25 | 56 |
3.3. Corrosion rates of metals
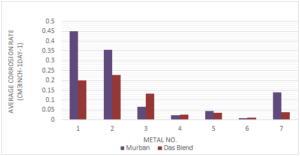
According to the determinations of weight loss method the average corrosion rates of metal coupon are given in the Figure 2.Figure 2. Corrosion rates of metals in both crude oils
According to the distribution of the corrosion rates there were observed relatively higher rates of corrosion from different three types of carbon steels and relatively lower corrosion rates in stainless steels including the least corrosion rates in both crude oils from 321-MN:1.4 304-MN:1.9 (Stainless Steel). Also the Monel metal showed moderate corrosion rates in both crude oils. When referring the chemical compositions of stainless steels those metals were composed at least 12 chromium with sufficient amount of nickel and also the combination of nickel and chromium tend to be formed much useful corrosive protection film on the relevant metal itself [1, 4,5, 10]. Therefore, the stainless steels showed relatively lower corrosion rates in both crude oil than the corrosion rates of other metals.
Regarding the effect of crude oils on the rate of corrosion in seven different types of ferrous metals there were found higher corrosion rates from four types of metals in Murban crude oil than the Das Blend crude oil since Das Blend was ahead in corrosive properties because of the requirement of higher temperature for a proper "sulfidation" process [2, 8, 9].
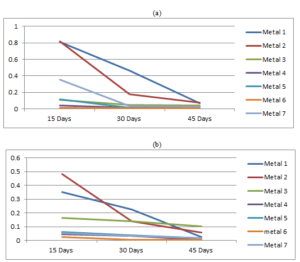
The variations of the corrosion rate of each metal coupon with the exposure time accordance to both crude oils are given in the Figure 3.Figure 3. Variation of the corrosion rates of metals with the exposure time in (a) Murban and in (b) Das Blend
3.4. Microscopic Review
According to the microscopic analysis there were identify some of distinguish properties of corrosion compounds based on their visible features foremost the color [4]. The important observations are highlighted in the Figure 4.Figure 4. Corroded surface of 410-MN: 1.8 420- MN: 2.8 (Stainless Steel) in Murban
- Ferrous Sulfides
- Corrosion Cracks
- Pitting Corrosion
- Trace Compounds
Table 4. Properties of corrosion compounds.
| Compound | Appearances | Observations |
|---|---|---|
| FeS | Black, brownish black, property of powder, pitting, cracks |
Observed most of features in |
| Fe\_2O\_3 | Rusty color | Observed rarely |
| Cus | Dark indigo/ dark blue | Unable to specify |
3.5. Decay of Metals
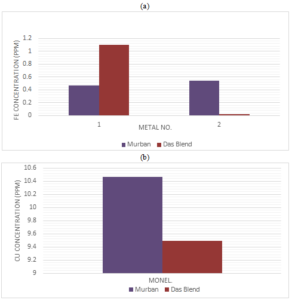
During the corrosion rate analysis there were found some invisible weight loss from most of metal coupons apart from specific corrosion compounds. The major objective of the analysis of atomic absorption spectroscopy (AAS) was to investigate the decay of metals into crude oils. The obtained results for the AAS analysis are shown in the Figure 5.Figure 5. (a) Ferrous concentration and (b) copper concentration in crude oil samples
3.6. Reduction of the Hardness in Metals
The distribution of the variations between initial hardness and hardness after corroded has been shown in the Figure 6.Figure 6. Variations of the initial hardness of metals in (a) Murban and in (b) Das Blend
4. Conclusion
According to the obtained results the least corrosion rates were found from 321-MN: 1.4 304-MN: 1.9 (Stainless Steel) in both crude oils while finding relatively corrosion rates from carbon steels and Monel as well. Because 321-MN: 1.4 304-MN: 1.9 (Stainless Steel) is composed 18 chromium and 8 nickel which is tend to create a stable corrosive protection layer on the metal surface itself. It was found higher corrosion rates of four types of metals in Murban crude oil while other three types were showing higher corrosion rates in Das Blend since Das Blend crude oil was composing higher amount of sulfur, mercaptans and organic acids although lower amount of salts than the Murban crude oil also it can be concluded the effect of salts is stronger than the overall effect of organic acids, sulfur and mercaptans at the room temperature. Apart from that there were identified black color corrosive compound, corrosion cracks and pitting corrosion accordance the microscopic analysis. Also found some significant ferrous concentrations in crude oil samples regarding carbon steels and significant amount of copper in crude oil samples regarding Monel since unable to find any amount of ferrous in crude oil samples regarding stainless steel. The slight reductions of the initial hardness of metal coupons were observed due to the corrosion eventually.Author Contributions
All authors contributed equally to the writing of this paper. All authors read and approved the final manuscript.Conflicts of Interest:
The authors declare no conflict of interest.References
- Khana, O.P. (2009). Materials Science and Metallurgy. New Delhi: Dhanpet Rai and Sons Publication.
- Fahim, M. A., Al-Sahhaf, T. A., & Elkilani, A. (2009). Fundamentals of petroleum refining. Elsevier. [Google Scholor]
- Oparaodu, K. O., & Okpokwasili, G. C. (2014). Comparison of percentage weight loss and corrosion rate trends in different metal coupons from two soil environments. International journal of environmental bioremediation & biodegradation, 2(5), 243-249. [Google Scholor]
- Callister, W. D. (2007). An introduction to materials science and engineering. John Wiley, New York, 1991) p, 420. [Google Scholor]
- Davis, M. E., & Davis, R. J. (2012). Fundamentals of chemical reaction engineering. Courier Corporation. [Google Scholor]
- Afaf, G. A. (2007). Corrosion Treatment of High TAN Crude (Doctoral dissertation, PhD. Thesis, University of Khartoum, Khartoum, Sudan).[Google Scholor]
- Badmos, A. Y., Ajimotakan, H. A., & Emmanuel, E. O. (2009). Corrosion in petroleum pipelines. New York science journal, 2(5), 36-40. [Google Scholor]
- Bolton, W. (2013). Engineering materials technology. Elsevier. [Google Scholor]
- Speight, J. G. (2014). The chemistry and technology of petroleum. CRC press. [Google Scholor]
- Singh, R. (2006). Introduction to Basic Manufacturing Process and Engineering Workshop. New Delhi: New Age International Publication. [Google Scholor]