Open Journal of Chemistry
ISSN: 2618-0758 (Online) 2618-074X (Print)
DOI: 10.30538/psrp-ojc2021.0019
Isolation and identification of Aspergilli causing Banana fruit rot
Fatima Ali\(^1\), Naureen Akhtar, Shazia Shafique, Sobiya Shafique
Division of Science and Technology, University of Education, Lahore-Pakistan.; (F.A & N.A)
Institute of Agricultural Sciences, University of the Punjab, Lahore-Pakistan.; (Shazia.S & Sobiya.S)
\(^{1}\)Corresponding Author; fatimahali4066@gmail.com
Abstract
Keywords:
1. Introduction
It is well known that fruits constitute commercially and nutritionally important indispensable food commodity. Fruits play an important role in human nutrition by supplying the necessary growth factors such as vitamins and essential minerals in human daily diet and that can help to keep a good and normal health. Fruits are widely distributed in nature. Banana (Musa paradisiaca L.) is an edible fruit, botanically a berry [1]. Banana plant is the largest herbaceous flowering plant [2], and its above ground parts arise from a corm [3]. Normally the plants are tall and firm with a false stem that is often mistaken to be a tree. Bananas usually grow in soils that are at least \(60\text{cm}\) deep, have a good drainage and are not compacted [3].
The banana develops from a dense hanging cluster that is made of tiers. Each tier usually contains \(20\) fruits. The hanging cluster of \(3-20\) tiers called a bunch can weigh \(30-50\) kilograms \((66-110\text{lb})\). Individual banana fruit weigh approximately \(125\) grams \((0.276\text{lb})\) of which \(75\%\) is water and \(25\%\) dry matter. Fruits may differ in size, color and firmness but mostly it is curved and long containing highly starch rich fresh and green, yellow or brown rind when ripe. Cultivated bananas differ in size depending upon their variety and growing conditions mostly \(5\text{m}\ \ (16\text{ft})\) tall, ranging from Dwarf Cavendish plants which are about \(3\text{m}\ \ (10\text{ft})\) tall to Gros Michell that are \(7\text{m}\ \ (23 \text{ft})\) tall [4]. Cultivated bananas are parthenocarpic; because they lack viable seeds, propagation involves removing and transplanting of corm by farmers.
As banana is a non-seasonal crop it is available round the year. The majority of all edible varieties developed from specific (inter- and intra-) hybridization of two seeded diploid Musa species (M. accuminata and M. balbisiana) and subsequent selection of diploid and triploid seedless clones. Despite rich genetic and phenotypic diversity, only a few clones developed, over time, into global commodities either as desert bananas, such as triploid Cavendish clones, or as important staple foods such as cooking bananas and plantains. Currently bananas are widely grown in tropics and are consumed in nearly all countries around the world, providing crucial nutrition for millions of people [5].
Bananas to be exported are picked when green and are ripened in special rooms on their arrival to destination countries. The rooms are designed to be airtight and are filled with ethylene to induce ripening of fruits. The vivid yellow color normally relates with the supermarket bananas and it is caused by artificial ripening process [6]. Ripening temperatures also affects the flavor and texture of bananas. During transport, bananas are refrigerated at \(13.5^0\)c to \(15^0\)C or \(56.3^0\)F to \(59.0^0\)F. At temperatures lower than this banana turn gray because of permanent stallation of ripening resulting in the breakage of cell walls.
Bananas are the fourth most important staple food in the world, after rice, wheat, and maize with more than \(100\) million tons produced annually [7]. Although the majority of bananas produced are consumed locally, banana export is a multi-dollar business [8]. Banana is a major fruit crop of Pakistan. It is grown on \(34,800\) hectares with production of \(154,800\) tons. It is mainly grown in Sindh province where the soil and climatic conditions are favorable for its successful cultivation. The total share of Sindh province alone in its cultivation is about \(85\%\) to \(92\%\) in banana acreage and about \(90\%\) of production.
Bananas are exported over long distances from the tropics to world markets. To prolong the shelf life, fruit is harvested before it matures. Storage and transport of bananas requires careful handling, rapid transport to ports, refrigerated shipping and cooling. The major goal during storage and transport is to prevent the bananas from producing its natural ripening agent, ethylene. This allows the storage and transport to be elongated for \(3 - 4\) weeks at \(13^0\) C or \(55^0\) F. One of the limiting factors that influence the fruit's economic value is the relatively short shelf life period caused by pathogens attacked. It is estimated that about \(20\%-25\%\) of the harvested fruits are decayed by pathogens during post-harvest handling even in developed countries. In developing countries, post-harvest losses are often more severe due to inadequate storage and transportation facilities [9].
Banana fruit is attacked by various pathogens either bacteria, viruses or fungi during their growing seasons, harvesting, handling, transport and post-harvest storage and marketing conditions, or after purchasing by the consumer. Fruits contain high levels of sugars and nutrient elements and their low pH values make them particularly desirable to fungal decay. Toxigenic fungi have been isolated from spoiling fruits. During refrigeration, some moulds may produce mycotoxins. Pathogenic fungi on the other hand, could cause infections or allergies. Aspergillus spp. is known to produce several toxic metabolites such as malformins, naphthopyrones and they can produce ochratoxins (OTA).
2. Objectives
The proposed objectives of the present study were as follows;- 1. Exploring the mycoflora associated with banana fruit rot;
- 2. Morphological and molecular characterization of isolated fungal pathogens toprovide authentic identification of species using polyphasic taxonomic approach;
- 3. Detection of new and unreported fungal pathogen(s) from Pakistan;
- 4. Determination of phylogenetic relationships of different species belonging to the same or different taxonomic groups of fungi.
3. Materials and methods
3.1. Sampling of diseased Banana fruits
Infected banana fruits were selected for the present study. About \(10-15\) samples were collected randomly from local markets of Lahore during January-February, \(2017\). Initially, infected fruits were examined physically and then brought to the laboratory in pre-sterilized sealed polythene bags, and kept in the refrigerator for further study. For each sample, records of sampling sites and data were maintained.3.2. Isolation and purification of Pathogens
Isolations of fungi from diseased fruits were carried out on Malt Extract Agar (MEA) medium. MEA was prepared by dissolving \(20\text{g}\) malt extract and \(20\text{g}\) agar in \(1000\text{mL}\) of distilled water. pH of fungal growth medium was adjusted to \(6.5\), followed by sterilization at \(121^0\)C and \(15lb/\text{inch}^2\) for \(15\) minutes. To the sterilized medium that was cooled to \(40- 45\), streptomycin at the rate of \(200\text{mg}\) per liter medium was added to avoid bacterial growth. Approximately, \(25\text{mL}\) medium was poured into each pre-sterilized petri plate. The medium in each petri plate was gently swirled and allowed to solidify at room temperature. Isolations from different samples were performed by either direct transfer of visible spores or transferring a small fragment of infected fruit part to the growth medium. Inoculated petri plates were incubated at \(25+2^0\)C for \(3-4\) days. Emerging colonies were purified by sub-culturing the spores from actively growing colonies onto the fresh MEA plates. Pure cultures of all the isolates were preserved for identification at \(4^0\)C.3.3. Identification and characterization of pathogens
Isolates were initially identified morphologically. Such phenotypic identification was confirmed by nucleotide sequence analysis of Internal Transcribed Spacer (ITS) sequence of rDNA.3.3.1. Morphological characterization
For morphology based identification macroscopic and microscopic observations were made using \(7\) days old pure fungus cultures grown at \(25+2^0\)C on MEA growth medium. Further to this, cultural and microphotographs were taken for the use of future reference and comparison in fungus identification. Macroscopic characters include colony color, size, shape, texture, margin zonation studied via naked eye and microscopic characters include conidiophores length, wall, vesicle size, and shape (if present), ornamentation of conidia, conidial size, shape and color etc. For microscopic examination of each fungal species, small pieces of fungal mycelium were placed in a drop of magnification of a calibrated compound microscope. Complete description of each isolate based on macro and micro morphological characters was prepared. Species were key out by comparing its description with published authentic literature [10,11,12,13].3.3.2. Molecular characterization
In this study, amplification of Internal Transcribed Spacer (ITS) region of ribosomal RNA was carried out using universal primer pair. Amplified gene products were sent for nucleotide sequencing and resulting DNA sequence were analyzed using bio informational tools.3.3.3. Genomic DNA extraction
Approximately \(250-300\text{mg}\) of freshly grown fungal cells was grounded into fine powder in a sterile pestle and mortar with the help of liquid nitrogen. The powdered cells were suspended in \(2\text{mL}\) Nucleon reagent \(\text{B}\)( \(400 \text{mM}\) tris \(\text{pH} 8\), \(120 \text{mM}\) EDTA \(\text{pH} 8, 150 \text{mM}\) NaCl and 1% (w/v) SDS containing \(0.5 \mu \text{L}\) of \(10 \text{mgmL}^{-1}\) RNase A in a sterile tube to nullify the RNA contamination in extracted DNA incubated at \(37^0\)C for \(30\) minutes. From the stock of \(5\text{M}\) Sodium perchlorate solution, \(0.5 \text{mL}\) was added to cell mixture and mixed thoroughly by inverting the tube several times. An aliquot of \(2 \text{ml}\) of ice chilled chloroform (at \(-20^0\)C) was added and mixed vigorously. The tube was then centrifuged at \(4000 \text{rpm}\) for \(5\) minutes. The supernatant was carefully transferred to a new sterile tube and \(2 \text{mL}\) of ice cold \(96\%\) ethanol was added. This tube was again centrifuged at \(4000 \text{rpm}\) for \(5\) minutes. Precipitated DNA pellets were collected and washed with \(70\%\) ethanol. Air dried DNA pellet was suspended in \(50 \mu \text{L}\) TE buffer (\(10\text{mM}\) tris, \(0.1\text{mM}\) EDTA, and \(\text{pH} 8\)) and incubated at \(65^0\)C for \(15\) minutes to inhibit the potential DNAse activity.3.3.4. Agarose gel electrophoresis for DNA quality analysis
Quality of extracted fungal DNA was analyzed through agarose gel electrophoresis. Agarose gel \((1\%)\) was prepared in 1X solution of TAE buffer from its \(50X\) stock (\(2\text{M} (242\text{g})\) of tris base in \(750\text{mL}\) of deionized water, \(1\text{M} (57.1\text{mL})\) of glacial acetic acid, \(100 \text{mL}\) of \(0.5 \text{M}\) EDTA by raising the volume \(1\) liter, \(pH 8.5\). Agarose \((1\text{g})\) was dissolved in \(100 \text{mL} 1\text{X}\) TAE by heating in a microwave oven. \(3\mu l\) Ethidium bromide from the stock of \(10 \text{mg}/\text{m}l\) was added to an agarose solution and mixed well. Gel was poured into the mold by avoiding air bubbles. Suitable comb was set to make wells for loading DNA samples. The gel was allowed to set completely for \(30-45\) minutes at room temperature. After solidification, the comb was removed carefully avoiding the tearing of wells. The gel casting tray was placed horizontally in an electrophoresis tank, containing an appropriate amount of \(1\text{X}\) TAE buffer. Gel loading dye (\(\mu \text{L}\) from \(6\text{X}\)) was added to each DNA sample. Samples were loaded carefully along with the DNA size marker. DNA samples were electrophoresed at \(100\) V for \(45\) minutes or until the dye migrated one third of the gel. DNA bands were visualized using a UV transilluminator and compared with the standard DNA size marker for quality estimation3.3.5. Amplification of ITS region of rDNA
The internal transcribed coding region of genome was amplified by using the genomic DNA template. Taq polymerase with appropriate buffer was used for amplification in \(25 \mu \text{L}\) PCR reaction mixture (\(12.25 \mu \text{L ddH}_2O; 1.5 \mu \text{L}\) of \(15 \text{mM MgC}l2; 2.5 \mu \text{L}\) dNTPs; \(2.5 \mu \text{L} 10\text{X}\) Taq buffer; \(0.5 \text{mL}\) of each primer, \(ITS1\) forward (\(5\)'-TCC GTA GGT GAA CCT GCG G-\(3\)') and \(\text{ITS}4\) reverse primer (\(3\)'-TCC TCC GCT TAT TGA TAT GC-\(5\)'), \(0.25 \mu \text{L}\) of Taq polymerase and \(5 \mu \text{L}\) of genomic DNA per reaction.The PCR reaction was carried out according to the following program. Amplification consisted of an initial denaturation step at \(94^0\)C for \(5\) minutes followed by \(35\) cycles each consisted of denaturation at \(94^0\)C for \(30\) seconds, annealing at \(55^0\)C for \(30\) seconds and amplification at \(72^0\)C for \(30\) seconds, with final extension at \(72^0\)C for \(10\) minutes. Amplified product was analysed on \(1\%\) agarose gel (\(5 \mu L\) of PCR product and \(1 \mu \text{L}\) of \(6\text{X}\) gel loading dye) by electrophoresis in \(1\text{X}\) TAE buffer and visualized under the UV transilluminator.
3.3.6. Analysis of nucleotide sequence by Bioinformatics tools
The nucleotide sequence of PCR product were analysed by Basic Local Alignment Search Tool (BLAST) on the NCBI and EBI (National Centre for Biotechnology Information and European Bioinformatics Institute respectively website and matched the similarity of amplified product nucleotides with NCBI and EBI data.4. Results
In the present study, four fungal pathogens were isolated from the rotten fruits of banana. These pathogens include Aspergillus niger, Aspergillus fumigatus and Aspergillus flavus. These isolates were identified initially on the basis of their morphological characters and then genetic analysis was carried out to confirm morphology based identifications.4.1. Morphology based Identifications
For morphological identifications, a comprehensive study of colonies as well as microscopic characteristics of all the strains was made and then compared with standard and authentic literature. All morphological observations were carried out on \(7\) days old pure fungus cultures grown at \(37^0\)C on MEA. Sporulation patterns, conidiophores and conidial morphology were examined under compound microscope (Labomed CX22; Labo America, Inc. USA).4.1.1. Aspergillus niger [Nyongesa et al., Advances in microbiology, pp. 205-229, (2000)]
Macroscopic features
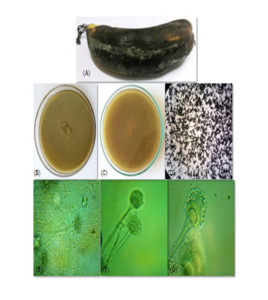
Colony on MEA was growing rapidly reaching \(3-3.5\) cm in diameter. Color of the colony was grey to black from the front while black at the reverse side. Colony texture was powdery due to heavy sporulation and distinct growth zones were present (Figure 1(B) &(C)). Visual examination of mycelia suggested that hyphae were initially yellow which turned to black with the formation of conidia or upon maturity of the colony. Under stereoscope conidial heads were strictly radiate (Figure 1(D)).Microscopic features
Conidiophores were hyaline, thick and smooth walled, long and globose at tip, usually \(100-135 \times 4-8 \mu m\) in size. Vesicles are sub-globose with an average diameter of \(10-25 \mu m\) (Figure 1 E & F). Conidial heads were biseriate with the phialides about \(8-10 \mu m\). Conidia were sub-globose to globose, and were greyish black as matured, produced in chains. Mature conidia ranged in size from \(3-5 \mu m\) (Figure 1(G)). The spore wall was smooth. Based on morphological characteristics, the pathogen was identified as Aspergillus niger.Figure 1. Aspergillus niger. (A) Infected Banana Sample; (B) Front; (C) reverse of colony grown on MEA; (D) Conidial heads under stereoscope; and (E-G) Microphotograph of conidial heads at 10X, 40X and 100X magnification of microscope, respectively.
4.1.2. Aspergillus fumigatus [Van Tieghem, in Ann. Sci. Nat. Botan., Ser. 5, 8: 240 (1867)]
Macroscopic Features
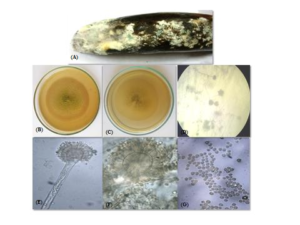
Colony on MEA spread rapidly and achieved a diameter of \(5-6\text{cm}\). Color of the colony was green at the front and off white to yellow at the reverse (Figure 2(B)&(C)). Margins of the colony were regular and no growth zones were observed. Texture of the colony was powdery due to extensive sporulation. Under stereoscope conidial heads were small and mostly radial (Figure 2(D)). At some positions conidial heads appeared columnar.Macroscopic Features
Hyphae were septate and hyaline. Conidiophores were short, long and cylindrical, coarsely roughened with conical terminal vesicle and ranged in size from \(15-25 \mu \text{m}\) (Figure 2(E) &(F)). Conidiophores usually range in size from \(200-215 \times 3-7 \mu \text{m}\). Conidial heads were uniseriate which supported a single row of phialides about \(10-12 \mu \text{m}\) in size on the upper two thirds of the vesicle. Conidia were smooth walled, globose, green in color, with an average size of \(2-4 \mu \text{m}\) (Figure 2(G)). Based on morphological features, the species was identified as Aspergillus fumigatus.Figure 2. Aspergillus fumigatus. (A) Infected Banana Sample; (B) Front; (C) reverse of colony grown on MEA; (D) Conidial heads under stereoscope; and (E-G) Microphotograph of conidial heads at 10X, 40X and 100X magnification of microscope, respectively.
4.1.3. Aspergillus flavus [Link, in Observations, pp. 16 (1809)]
Macroscopic features
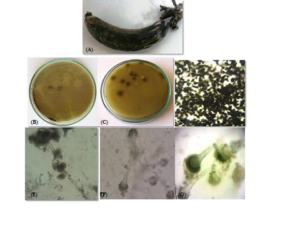
Colony was growing rapidly attaining the diameter of \(5-6 c\text{m}\) when grown on MEA. Colony was heavily sporulated, powdery in texture, light to bright green in color and olive green on the reverse side (Figure 3(B)&(C)). Vegetative mycelia were white. Growth zones were absent. Under stereoscope conidial heads were prominent and radial (Figure (D)).Macroscopic features
Hyphae were septate and hyaline that formed dense mycelia. Conidiophores were hyaline, coarsely roughened with an average size of \(170 \times 15 \mu \text{m}\). Conidial heads were uniseriate with phialides mostly \(10-18 \mu \text{m}\) size and born directly on the vesicle. Vesicles were hyaline, subglobose to globose with an average diameter of \(25-30 \mu \text{m}\) (Figure 3(E)&(F)). Conidia were sub-globose to globose and yellowish green in color that arranged in size from \(3-6 \mu \text{m}\) (Figure 3(G)). Based on morphological features, the species was identified as Aspergillus flavus.Figure 3. Aspergillus flavus. (A) Infected Banana Sample; (B) Front; (C) reverse of colony grown on MEA; (D) Conidial heads under stereoscope; and (E-G) Microphotograph of conidial heads at 10X, 40X and conidia at 100X magnification of microscope, respectively
4.1.4. Aspergillus fumigatus [Thom and Raper, Manual of the Aspergilli, pp. 148-151, (1945).]
Macroscopic features
Colony was bright green in color and pale green on the reverse side. Texture of the colony was powdery and diameter on MEA was \(4.5-5 \text{cm}\) (Figure 4(B)&(C)). Conidial heads were columnar when studied under stereoscope (Figure 4(D)).Microscopic features
Conidiophores were hyaline, thick and smooth walled having an average size \(180 \times 17 \mu \text{m}\). Hyphae were septate and hyaline. Both uniseriate and biseriate conidial heads were present. Vesicles were sub-globose that ranged in size from \(11-15 \mu \text{m}\) (Figure 4(E) &(F)). Conidia were globose, pale green in color, \(2-4 \mu \text{m}\) in diameter. The wall of conidia was smooth however some geniculations were observed (Figure 4(G)). Based on morphological features the species was identified as Aspergillus fumigatus.Figure 4. Aspergillus fumigatus. (A) Infected Banana Sample; (B) Front; (C) reverse of colony grown on MEA; (D) Conidial heads under stereoscope; and (E-G) Microphotograph of conidial heads at 10X, 40X and conidia at 100X magnification of microscope, respectively.
4.2. Molecular analysis
In the present studies, genetic analysis of isolated fungal species was performed by nucleotide sequence analysis of ITS region of rDNA.4.2.1. Genomic DNA extraction of fungal species
Total four isolated fungal strains were subjected to DNA extraction. The isolated DNA samples were run on \(1\%\) agarose gel. For all strains, a compact single band of about \(13000 \text{bp}\) was observed on gel. The DNA bands were compared with standard DNA marker and equalized by eye for easy quantifications. Results of all the isolates are shown in Figure 5.Figure 5. Genomic DNA extraction from different fungal species isolated from rotten banana fruits
4.2.2. Results of internal transcribed spacer (ITS) sequencing
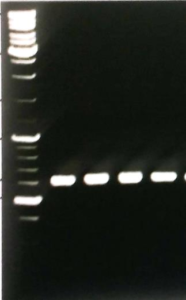
The consensus primers \(\text{ITS}1\) and \(\text{ITS}4\) were used for the amplification of Internal Transcribed region. Fungus-specific universal primer pairs (\(\text{ITS}1\) and \(\text{ITS}4\)) were able to successfully amplify the \(\text{ITS}1-5.8\text{S}\) rDNA-\(\text{ITS}4\) region of all the tested isolates, providing a single PCR product of about \(500 \text{bp} - 650 \text{bp}\) (Figure 6). Purified PCR products yielded sequences of \(500-595 \text{bp}\) in length. Using the National Centre for Biotechnology Information (NCBI) and European Bioinformatics Institute (EBI) bioinformatics websites DNA sequences were BLAST. Fungal species were selected after DNA sequence blast with maximum similarity percentage \((95-100\%)\).Figure 6. DNA fragments of fungal species amplified by primer ITS1 and ITS4.
3. Blast results
The ITS sequence alignment of two different isolates of Aspergillus fumigatus showed \(99\%\) homology to \(004\ \ (\text{KU}321562.1),\ \ \text{SK}1 \ \ (\text{KM}207771.1),\ \ \text{CD}1621 \ \ (\text{JX}092088.1),\ \ \text{DS-C}3\ \ (\text{HQ}285554.1),\ \ \text{LR}7 \ \ (\text{KM}520022.1)\) and \(98\%\) identity to \(\text{AHBR}16\ \ (\text{KF}305755.1),\ \ \text{SF}8\ \ (\text{KX}011021.1)\) (Figures 7 and 8).
Figure 7. ITS region DNA sequence alignment of Aspergillus fumigatus.
The BLAST results revealed \(99\%\) identity of Aspergillus fumigatus to the strains \(004\ \ (\text{K}4321562.1),\ \ \text{SK}1\ \ (\text{KM}207771.1),\ \ \text{Cd}1621\ \ (\text{JX}092088.1)\) and \(\text{DS-C}3\ \ (\text{HQ}285554.1)\).
Figure 8. ITS region DNA sequence alignment of Aspergillus fumigatus.
The BLAST results revealed \(99\%\) identity of Aspergillus fumigatus to the strains \(\text{LR}7\ \ (\text{KM}520022.1)\) and \(98\%\) to the strains \(\text{SK}1\ \ (\text{KM}207771.1),\ \ \text{AHBR}16 \ \ (\text{KF}305755.1),\ \ \text{Sf}8\ \ (\text{KX}011021.1)\).
The present study was aimed to isolate and identify economically important fungal strains using polyphasic approaches comprising of morphology and genetics. Aspergillus species are known to cause infection in variety of fruits in addition to banana i.e., citrus fruits, tomato, peach, mango etc. Aspergillus flavus and Aspergillus fumigatus causing tomato spoilage was also investigated by [15]. Similarly, peach and oranges had been studied for fungal decay in storage and a number of Aspergillus species including Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger and Aspergillus candidus had been isolated [16]. Aspergillus niger was also isolated from spoiled ripe mango by Palwjwala et al., [17].
The consensus primers \(\text{ITS}1\) forward (\(5’-\text{TCC GTA GGT GGA CCT GCG G-}3’\)) and \(\text{ITS}4 \ \ (5’\text{- TCC TCC GCT TAT TGA TAT GC - }3’)\) were used for the amplification of Internal transcribed region. The small variations in the band size probably makes ITS an unreliable parameter for separating fungal species [18]. The uniformity of ITS size in different fungal groups makes nucleotide sequencing of ITS fragment necessary to reveal interspecific and in some cases, also intraspecific variations.
Resulting nucleotide sequences were analyzed using National Centre for Biotechnology Information (NCBI) and European Bioinformatics Institute (EBI) bioinformatics websites. The fungal species finalized after DNA sequence blast with maximum similarity percentage \((97-100\%)\). Two species of Aspergillus fumigatus has been \(98\%\) and \(99\%\) similarity with DNA sequence when blasted and pre-identified on the basis of morphology.
Fungal isolations are very helpful in the fields of fungal biotechnology that plays a major role for many industries including food and feed, pharma, paper and pulp etc. For example, the worldwide production of citric acid produced using filamentous fungi Aspergillus niger by far exceeds the production of any other organic acid made by microbial fermentation [19].
The identification of these isolates was not an easy task due to morphological similarities amongst the different isolates of a genus. Molecular tools like ITS provided further authenticity to the identification. Therefore, polyphasic taxonomic approaches adopted in current investigation facilitated to achieve a reliable identification of four isolates with different sections of fungi that will indeed aid great help in different areas of research and teaching.
5. Conclusion
Four strains of fungi were isolated from rotten banana fruits and were preliminary identified on morphological basis. Identified species were two of Aspergillus fumigatus, one each of Aspergillus flavus and Aspergillus niger.BLAST results using ITS sequence of all identified strains showed \(97-100\%\)identity with many of their respective strains deposited to GenBAnk. Phylogenetic analysis of ITS based results revealed lack of clear distinction amongst phylogenetically similar isolates.
Author Contributions
All authors contributed equally to the writing of this paper. All authors read and approved the final manuscript.Conflicts of Interest
The Authors declare that there is no conflict of interest.References
- Morton, J. F. (1987). Fruits of Warm Climates. JF Morton.[Google Scholor]
- INIBAP, P. C. (2000). Bananas (English ed.). Montpellier: International Network for the Improvement of Banana and Plantains/International Plant Genetic Resources Institute. Retrieved 2013-01-31. [Google Scholor]
- Stover, R. H., & Simmonds, N. W. (1987). Bananas(No. Ed. 3). Longman Scientific & Technical. [Google Scholor]
- Ploetz, R. C., Kepler, A. K., Daniells, J., & Nelson, S. C. (2007). Banana and plantain—an overview with emphasis on Pacific island cultivars. Species profiles for Pacific Island Agroforestry, 1, 21-32. [Google Scholor]
- Ordonez, N., Seidl, M. F., Waalwijk, C., Drenth, A., Kilian, A., Thomma, B. P., ... & Kema, G. H. (2015). Worse comes to worst: bananas and Panama disease--when plant and pathogen clones meet. PLoS Pathogens, 11(11), Article ID: e1005197. [Google Scholor]
- Hassan, I., Chattha, M. B., Chattha, T. H., & Ali, M. A. (2010). Factors affecting wheat yield: a case study of mixed cropping zone of Punjab. Journal of Agricultural Research, 48(3), Article No. 403. [Google Scholor]
- Churchill, A. C. (2011). Mycosphaerella fijiensis, the black leaf streak pathogen of banana: progress towards understanding pathogen biology and detection, disease development, and the challenges of control. Molecular Plant Pathology, 12(4), 307-328. [Google Scholor]
- Marin, D. H., Romero, R. A., Guzmán, M., & Sutton, T. B. (2003). Black Sigatoka: an increasing threat to banana cultivation. Plant Disease, 87(3), 208-222. [Google Scholor]
- Al-Hindi, R. R., Al-Najada, A. R., & Mohamed, S. A. (2011). Isolation and identification of some fruit spoilage fungi: Screening of plant cell wall degrading enzymes. African Journal of Microbiology Research, 5(4), 443-448. [Google Scholor]
- Thom, C., & Raper, K. B. (1945). A Manual of the Aspergilli (Vol. 60, No. 4, p. 333). LWW. \bibitem{dw}Domsch, K. H., Gams, W., & Anderson, T. H. (1980). Compendium of Soil Fungi. Volume 1. Academic Press (London) Ltd. [Google Scholor]
- Domsch, K. H., Gams, W., & Anderson, T. H. (1980). Compendium of Soil Fungi. Volume 1. Academic Press (London) Ltd. [Google Scholor]
- Manoharachary, C., Rao, N. K., Agarwal, D. K., & Kunwar, I. K. (2006). Two new species of Acrodictys MB Ellis from India. Indian Phytopathology, 59(1), 91-93. [Google Scholor]
- Manamgoda, D. S., Cai, L., Bahkali, A. H., Chukeatirote, E., & Hyde, K. D. (2011). Cochliobolus: an overview and current status of species. Fungal Diversity, 51(1), 3-42. [Google Scholor]
- Nyongesa, B. W., Okoth, S., & Ayugi, V. (2015) Identification Key for Aspergillus Species Isolated from Maize and Soil of Nandi County, Kenya. Advances in Microbiology, 5, 205-229. [Google Scholor]
- Adisa, V. A. (1985). Some extracellular enzymes associated with two tomato fruit spoilage molds. Mycopathologia, 91(2), 101-108. [Google Scholor]
- Singh, D., & Sharma, R. R. (2007). Postharvest Diseases of Fruit and Vegetables and Their Management In: Prasad (Doctoral dissertation, D.(Ed). Sustainable Pest Management, Daya Publishing House, New Delhi. India, 87-98p). [Google Scholor]
- Palejwala, V. A., Patki, C. K., Bhatt, S. V., & Modi, V. V. (1987). Post-harvest spoilage of mangoes by Aspergillus niger. International Journal of Food Microbiology, 5(2), 111-116. [Google Scholor]
- Hinrikson, H. P., Hurst, S. F., Lott, T. J., Warnock, D. W., & Morrison, C. J. (2005). Assessment of ribosomal large-subunit D1-D2, internal transcribed spacer 1, and internal transcribed spacer 2 regions as targets for molecular identification of medically important Aspergillus species. Journal of clinical microbiology, 43(5), 2092-2103. [Google Scholor]
- Meyer, R., Köhler, J., & Homburg, A. (2016). Explosives. John Wiley & Sons. [Google Scholor]