Trends in Clinical and Medical Sciences
ISSN: 2791-0814 (online) 2791-0806 (Print)
DOI: 10.30538/psrp-tmcs2021.0005
A prospective study for assessment of Serum Adenosine Deaminase, Erythrocyte Sedimentation Rate and C-Reactive Protein levels in patients with Psoriasis
Leo Tauro\(^1\), Nutsukpo Amankwa
Department of Dermatology, National University of Ghana, Legon, Ghana.; (L.T & N.A)
\(^{1}\)Corresponding Author: drleofrancis.reviewer@gmail.com
Abstract
Keywords:
1. Introduction
Psoriasis is a common chronic inflammatory skin disease and is primarily characterized by localized or generalized scaly erythematous plaques. The rapid proliferation of epidermal keratinocytes in the basal layer is the primary pathological characteristic of psoriasis. Psoriasis is a disease of the immune system [1]. Not only it can affect the skin and joints, but it is also associated with metabolic syndrome, obesity, cardiovascular disease, and mental diseases. Furthermore, psoriasis can result in bodily harm to the patient as well as impacts patients' appearance, social activities, and quality of life [2].
Various studies have shown elevated ADA activity in epidermis of psoriasis patients. However, this could be due to increased nucleic acid catabolism associated with the hyperproliferative status of epidermis in psoriasis patients [3]. The epidermis of psoriatic patients showed high levels of ADA which correlated with the hyperproliferative states of the keratinocytes with pronounced DNA synthesis [4]. In addition, plasma ADA activity was higher in psoriatic patients compared to controls and decreased after treatment with propylthiouracil (PTU), PUVA, or cyclosporine [5]. ADA regulates the plasma adenosine level, which is directly or indirectly involved in inflammatory molecules and cytokine production. The ambiguous nature of ADA gives insight into its involvement in various disorders [6].
C-reactive protein (CRP) is an important laboratory parameter for tissue damage, infection, and inflammation [7]. High-sensitive CRP (hsCRP) can detect lower levels of CRP than the standard CRP measurement. Increased hsCRP is found in many skin diseases including allergic contact dermatitis, mycosis fungoides, hidradenitis suppurativa, and psoriasis [8]. Increased CRP in psoriatic patients was correlated with active arthritis, psoriasis area severity index (PASI) score, and with an increased incidence of cardiovascular diseases [9]. Patients with increased hsCRP levels were found to have a better response to cyclosporine therapy [10]. Considering this, we attempted this study to assess serum ADA, ESR and CRP level in patients with Psoriasis.
2. Methodology
This observation study was conducted among sixty- four patients with psoriasis. Approval for the study was obtained from ethical review and clearance committee. All patients gave their written consent for the part of the study.All patients were asked for drug history, and history of medical diseases with stress on age, duration, and joint affection. Patients were divided into three groups (mild, moderate, and severe) based on PASI scores. PASI score < 10 defined psoriasis as mild, between 10 and 20 as moderate, and >20 as severe. Patients (Group I) were compared with control (Healthy) (Group II). From each subject, 8 ml venous blood sample was withdrawn for the assessment of CRP and ADA. The hsCRP was assayed by an enzyme-linked immunosorbent assay BIOS kit. ADA was measured through kinetic method. ESR was calculated using Westergren method. Results of the present study after recording all relevant data were subjected for statistical inferences using chi- square test. The level of significance was significant if p value is below 0.05 and highly significant if it is less than 0.01.
3. Results
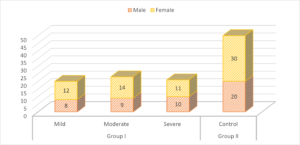
Group I patients had 8 males (mild), 9 males (moderate) and 10 males (severe) and group II had 20 males. There were 12 females (mild), 14 females (moderate) and 11 females (severe) and group II had 30 females. A non- significant difference was observed (\(P> 0.05\)) (Table 1, Figure 1).
Table 1. Characteristics of subjects.
| Variables | Group I | Group II | P value | ||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | Control | ||
| Male | 8 | 9 | 10 | 20 | 0.05 |
| Female | 12 | 14 | 11 | 30 | |
Figure 1. Characteristics of subjects
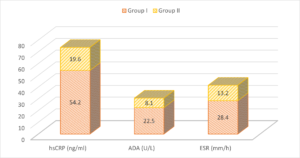
The mean hSCRP level in group I patients was 54.2 ng/ml and in group II was 19.6 ng/ml. The mean ADA level in group I patients was 22.5 U/L and in group II was 8.1 U/L. The mean ESR was 28.4 mm/h in group I and 13.2 mm/h in group II. A significant difference was observed (\(P< 0.05\)) (Table 2, Figure 2).
Table 2. Assessment of parameters.
| Parameters | Group I | Group II | P value |
|---|---|---|---|
| hsCRP (ng/ml) | 54.2 | 19.6 | <0.05 |
| ADA (U/L) | 22.5 | 8.1 | <0.05 |
| ESR (mm/h) | 28.4 | 13.2 | <0.05 |
Figure 2. Assessment of parameters
4. Discussion
This case control study was conducted among 64 cases and 50 control subjects and the level of ADA, hSCRP and ESR was calculated and compared [11] Psoriasis is a chronic systemic disease with an immune-inflammatory etiology, affecting approximately 2%-3% of the world's population, and characterized by T-cell-mediated hyperproliferation of keratinocytes [12].Psoriasis is a chronic skin disorder, with extensive systemic involvement of an unknown etiology [13]. However, the most accepted hypothesis is that it has an immunological involvement due to its association with certain human leukocyte-associated antigens, presence of activated T lymphocytes in lesions, and its response to immunosuppressive therapies [14]. Adenosine deaminase (ADA) is an enzyme involved in purine metabolism and is essential for the breakdown of adenosine from food and the turnover of nucleic acids in tissues. It is considered as a marker of nonspecific T-cell activation. ADA (EC 3.5.4.4) is an enzyme that catabolizes purine nucleotides [15]. It is involved in the hydrolytic deamination of adenosine and 2-deoxyadenosine to inosine and 2-deoxyinosine, respectively. The role of ADA in function and maturation of lymphoid cells, especially T-cell lineage, and its altered status in diseases with immunological disturbances have proven to be very crucial and informative [16].
Our study demonstrated that there were 8 males in mild, 9 males in moderate and 10 males in severe form and 20 males in group II. There were 12 females in mild, 14 females in moderate and 11 females in severe form and 30 females in group II. Moustafa et al., [17] included 60 psoriatic patients which were divided according to PASI score into three groups (mild, moderate, and severe) each containing 20 patients. Twenty healthy subjects of matched age and sex were included as control. Serum ADA, hsCRP, SUA, and ESR were evaluated for patients and controls. While ADA, hsCRP, SUA, and ESR showed a significant increase in psoriatic patients compared with that of the controls (P0.05) and no correlations with PASI score \((P>0.05)\). The frequency of joint affection increased with increasing severity of psoriasis (5%, 10%, and 25% in mild, moderate, and severe psoriasis, respectively).
We observed that the mean hSCRP level in group I patients was 54.2 ng/ml and in group II was 19.6 ng/ml. The mean ADA level in group I patients was 22.5 U/L and in group II was 8.1 U/L. The mean ESR was 28.4 mm/h in group I and 13.2 mm/h in group II. Nigam et al., [17] found no significant difference between SUA of psoriatic patients and healthy population and reported a positive correlation of SUA with PASI score. Kose et al., [18] noticed decreased ADA activity in plasma and skin tissue from baseline value after treatment of psoriasis for 2 months. Bukulmez et al., [19] found predominantly elevated ADA level in 25 psoriasis patients. The increase in ADA activity might be an indicator of activation of the immune system that might have originated from activation of T-lymphocytes in these patients. In addition, no previous reports concerning ADA activity in psoriasis patients have revealed a correlation between PASI scores and ADA activity.
hs-CRP is an acute phase reactant which is raised in many inflammatory conditions. Its level can increase up to many folds within 24 hours and decrease to normal in a few days after treatment or spontaneously decrease after remission of disease. Silva et al., [20] established a correlation between the psoriasis area and severity index (PASI) and the Dermatology Life Quality Index (DLQI) based on a quality of life questionnaire adapted to the Brazilian context for patients with plaque psoriasis before and after systemic treatment. Patients were evaluated according to the PASI and the quality of life questionnaire adapted to the Brazilian context before and 60 days after systemic treatment. Thirty-five patients participated in the study. Twenty-six were men, with a mean age of 46 years. There was no correlation between the PASI and the quality of life questionnaire adapted to the Brazilian context, but there was a correlation between the PASI and some items of the quality of life questionnaire adapted to the Brazilian context, such as jobs involving public contact.
There were few limitations of our study. First one small sample size and short follow up. Assessment of serum zinc, copper and serum uric acid was not performed.
5. Conclusion
Results of present study demonstrated higher hSCRP, ESR and ADA level among patients suffering from psoriasis compared to healthy control.Author Contributions
All authors contributed equally to the writing of this paper. All authors read and approved the final manuscript.Conflicts of Interest
The authors declare no conflict of interest.References
- Rifai, N., & Ridker, P. M. (2001). High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clinical Chemistry, 47(3), 403-411. [Google Scholor]
- Zinkeviciene, A., Kainov, D., Lastauskiene, E., Kvedariene, V., Bychkov, D., Byrne, M., & Girkontaite, I. (2015). Serum biomarkers of allergic contact dermatitis: A pilot study. International Archives of Allergy and Immunology, 168(3), 161-164. [Google Scholor]
- Cengiz, F. P., & Emiroglu, N. (2015). Evaluation of cardiovascular disease risk factors in patients with mycosis fungoides. Anais Brasileiros de Dermatologia, 90, 36-40. [Google Scholor]
- Miller, I. M., Ring, H. C., Prens, E. P., Rytgaard, H., Mogensen, U. B., Ellervik, C., & Jemec, G. B. (2016). Leukocyte profile in peripheral blood and neutrophil-lymphocyte ratio in hidradenitis suppurativa: a comparative cross-sectional study of 462 cases. Dermatology, 232(4), 511-519. [Google Scholor]
- Gerkowicz, A., Pietrzak, A., Szepietowski, J. C., Radej, S., & Chodorowska, G. (2012). Biochemical markers of psoriasis as a metabolic disease. Folia Histochemica et Cytobiologica, 50(2), 155-170. [Google Scholor]
- Beygi, S., Lajevardi, V., & Abedini, R. (2014). C-reactive protein in psoriasis: a review of the literature. Journal of the European Academy of Dermatology and Venereology, 28(6), 700-711. [Google Scholor]
- Siegel, D., Devaraj, S., Mitra, A., Raychaudhuri, S. P., Raychaudhuri, S. K., & Jialal, I. (2013). Inflammation, atherosclerosis, and psoriasis. Clinical Reviews in Allergy & Immunology, 44(2), 194-204. [Google Scholor]
- Ohtsuka, T. (2008). The correlation between response to oral cyclosporin therapy and systemic inflammation, metabolic abnormality in patients with psoriasis. Archives of Dermatological Research, 300(10), 545-550. [Google Scholor]
- Al Raddadi, A., Jfri, A., Samarghandi, S., Matury, N., Habibullah, T., Alfarshoti, M., & Mahdi, A. (2016). Psoriasis: Correlation between severity index (PASI) and quality of life index (DLQI) based on the type of treatment. Journal of Dermatology & Dermatologic Surgery, 20(1), 15-18. [Google Scholor]
- Gousia Sheikh, Q. M., Majeed, S., & Hassan, I. (2015). Comparison of levels of serum copper, zinc, albumin, globulin and alkaline phosphatase in psoriatic patients and controls: A hospital based casecontrol study. Indian Dermatology Online Journal, 6(2), 81-83. [Google Scholor]
- Burden-Teh, E., Thomas, K. S., Ratib, S., Grindlay, D., Adaji, E., & Murphy, R. (2016). The epidemiology of childhood psoriasis: a scoping review. British Journal of Dermatology, 174(6), 1242-1257. [Google Scholor]
- Zeng, Q., Yin, J., Fan, F., Chen, J., Zuo, C., Xiang, Y., ... & Xiao, R. (2014). Decreased copper and zinc in sera of Chinese vitiligo patients: A meta-analysis. The Journal of Dermatology, 41(3), 245-251. [Google Scholor]
- Basavaraj, K. H., Darshan, M. S., Shanmugavelu, P., Rashmi, R., Mhatre, A. Y., Dhanabal, S. P., & Rao, K. S. J. (2009). Study on the levels of trace elements in mild and severe psoriasis. Clinica Chimica Acta, 405(1-2), 66-70. [Google Scholor]
- Lei, L., Su, J., Chen, J., Chen, W., Chen, X., & Peng, C. (2019). Abnormal serum copper and zinc levels in patients with psoriasis: A meta-analysis. Indian Journal of Dermatology, 64(3), 224-230. [Google Scholor]
- Wang, G. Z., Yu, X. J., & Xiao, J. G. (2004). Changes of serum level of zinc in children with psoriasis vulgaris. Zhongguo Zhongxiyi Jiehe Pifu Xingbingxue Zazhi, 3, 144-146. [Google Scholor]
- Moustafa, Y. M., Elsaied, M. A., Abd-Elaaty, E. M., & Elsayed, R. A. (2019). Evaluation of serum adenosine deaminase and inflammatory markers in psoriatic patients. Indian Journal of Dermatology, 64(3), 207-212. [Google Scholor]
- Nigam, P. K. (2005). Serum zinc and copper levels and Cu: Zn ratio in psoriasis. Indian Journal of Dermatol Venereol Leprol, 71(3), 205-206. [Google Scholor]
- Köse, K., Utas, S., Yazici, C. E. V. A. T., Akdas, A., & Kelestimur, F. (2001). Effect of propylthiouracil on adenosine deaminase activity and thyroid function in patients with psoriasis. British Journal of Dermatology, 144(6), 1121-1126. [Google Scholor]
- BUKULMEZ, G., Tülin, A. K. A. N., & CILIV, G. (2000). Serum adenosine deaminase levels in patients with psoriasis: a prospective case-control study. European Journal of Dermatology, 10(4), 274-276.[Google Scholor]
- da Silva, M. F. P., Fortes, M. R. P., Miot, L. D. B., & Marques, S. A. (2013). Psoriasis: correlation between severity index (PASI) and quality of life index (DLQI) in patients assessed before and after systemic treatment. Anais Brasileiros de Dermatologia, 88(5), 760-763.[Google Scholor]