Trends in Clinical and Medical Sciences
ISSN: 2791-0814 (online) 2791-0806 (Print)
DOI: 10.30538/psrp-tmcs2021.0015
A comparative analysis of curcumin and clobetasol propionate in management of patients with oral lichen planus
Ruchi Tantia\(^1\), Ruchi Gupta
Department of Pharmacology, RNT Medical College, Udaipur, Rajasthan, India.; (R.T)
Consultant, Neuroderma Clinic, Dermatology Wing, Aligarh, India.; (R.G)
\(^{1}\)Corresponding Author: ruchi.jnmc@gmail.com
Abstract
Keywords:
1. Introduction
Oral lichen planus (OLP) is a chronic mucocutaneous, autoimmune and inflammatory disease affecting oral mucosa, skin and genitalia which is T-cell mediated [1,2]. It involves 0.1-2% of general population. It has malignant potential between 0.07% and 5.8%. It commonly affects females in their 4th decade of life and clinically appears as white striations, erosions and desquamative gingivae [3]. Various forms are reticular, erosive, erythematous, popular, plaque, bullous, annular and ulcerative [4]. Main pathogenesis of OLP is antigen specific cell mediated immune response, which includes accumulation of activated CD8+lymphocytes on basal keratinocytes causing apoptosis. Genetic, psychological factors initiate autoimmune mediated response [5].
Corticosteroids are the mainstay treatment modality and effective in controlling the symptoms of the disease; but side effects such as secondary candidiasis, telangiectasia, hypothalamic-pituitary-adrenal suppression, muco-cutaneous atrophy and increased potential of systemic absorption are also common with its prolonged use [6]. Curcuma longa is a perennial plant belonging to Zingiberaceae family possessing anti-inflammatory effects. Its main constituents include three curcuminoids including curcumin (the primary ingredient and the one responsible for its yellow color and anti-inflammatory effect), demethoxycurcumin and bisdemethoxycurcumin [7]. It has anti-oxidant, anti-carcinogenic, anti-microbial, anti-proliferative and wound healing properties. Studies show that curcumin reduces multiple sclerosis, rheumatoid arthritis, psoriasis, inflammatory bowel disease in human and animal models [8]. These properties have prompted investigators to check its efficacy on oral diseases namely, lichen planus, oral submucous fibrosis (OSMF), leukoplakia, recurrent aphthous stomatitis, etc. Previous studies have indicated that use of topical curcumin has a chemopreventive effect in oral potentially malignant disorders such as OSMF and leukoplakia [9,10]. Considering this, we attempted this study to compare curcumin and clobetasol propionate in management of patients with oral lichen planus.
2. Methodology
We included sixty- four patients of OLP in our prospective observational study with the informed verbal consent of all participants. Institutional ethical review committee's approval for the present study was obtained. Our study followed declaration of Helsinki. All cases were confirmed clinically as well as histopathologically and only atrophic and ulcerative variety was taken. The exclusion criteria were pregnant lactating mothers, patients on anticoagulants or antiplatelet agents, history of gastric ulcers, duodenal ulcers.A simple stratified random sampling was performed where 32 patients were put in group I in which patients received 0.1% triamcinolone and in group 2, 32 patients received 5% curcumin paste for 4 weeks and were asked to apply it TDS per day after eating and brushing. All patients were refrain from eating for 20 minutes. Measurement of the appearance score and severity of pain was done at baseline and at the end of 2 and 4 weeks and recorded in the patients' questionnaires. In our study we used the criteria by Thongprasom et al. for determining the appearance score as: 0: No lesion, normal mucosa, 1: Mild white striae, no erythematous area, 2: White striae with atrophic area less than \(1cm^{2}\),3: White striae with atrophic area more than \(1cm^{2}\) , 4: White striae with ulcerative area less than \(1cm^{2}\) and 5: White striae with ulcerative area more than \(1cm^{2}\). Response rate was measured as complete remission-100% reduction in signs and symptoms, good response- 50% or more reduction in signs and symptoms and poor response- < 50% reduction in signs and symptoms. Results were compared in two groups using Mann Whitney U test where p value less than 0.05 considered significant.
3. Results
The mean age was 45.2 years in group 1 and 43.7 years in group 2. Males: Females ratio of 10:22 and 11:21 was seen in group 1 and 2 respectively. Pain at baseline (VAS) was 5.48 in group 1 and 5.12 in group 2. At baseline, lesion size was 3.85 cm2 in group 1 and 3.72 \(cm^{2}\) in group 2, see Table 1.
Table 1. Baseline characteristics.
| Characteristics | Group 1 | Group 2 |
|---|---|---|
| Mean age (years) | 45.2 | 43.7 |
| M:F | 10:22 | 11:21 |
| Pain at baseline (VAS) | 5.48 | 5.12 |
| Lesion size at baseline (cm2) | 3.85 | 3.72 |
Mann Whitney U test
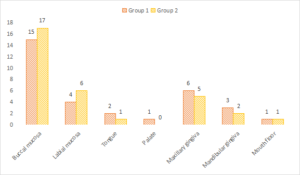
Common site was buccal mucosa in both groups (group 1- 15, group 2- 17), maxillary gingiva (group 1- 6, group 2- 5), labial mucosa (group 1- 4, group 2- 6), mandibular gingiva (group 1- 3, group 2- 2), tongue (group 1- 2, group 2- 1), floor of mouth (group 1- 1, group 2- 1) and palate (group 1- 1, group 2- 0). A non- significant difference among site distribution was seen (P> 0.05), see Table 2 and Figure 1.
Table 2. Common site involved in both groups.
| Site | Group 1 | Group 2 | P value |
|---|---|---|---|
| Buccal mucosa | 15 | 17 | >0.05 |
| Labial mucosa | 4 | 6 | |
| Tongue | 2 | 1 | |
| Palate | 1 | 0 | |
| Maxillary gingiva | 6 | 5 | |
| Mandibular gingiva | 3 | 2 | |
| Mouth floor | 1 | 1 |
Figure 1. Graphical representation of common site involved in both groups
Mann Whitney U test
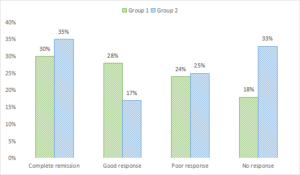
Complete remission in pain was seen in 30% in group 1 and 35% in group 2, good response in 28% in group 1 and 17% in group 2, poor response in 24% in group 1 and 25% in group 2 and no response in 18% in group 1 and 33% in group 2. A non- significant difference among good and no response was seen (P< .05), see Table 3 and 2.
Table 3. Comparison of pain reduction.
| Response | Group 1 | Group 2 | P value |
|---|---|---|---|
| Complete remission | 30% | 35% | >0.05 |
| Good response | 28% | 17% | < 0.05 |
| Poor response | 24% | 25% | >0.05 |
| No response | 18% | 33% | < 0.05 |
Figure 2. Graphical comparison of pain reduction
Mann Whitney U test
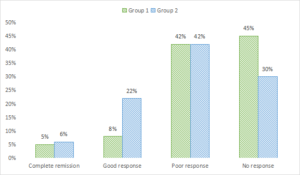
Complete remission in clinical response was seen in 5% in group 1 and 6% in group 2, good response in 8% in group 1 and 22% in group 2, poor response in 42% in group 1 and in group 2 and no response in 45% in group 1 and 30% in group 2. A non- significant difference among good and no response was seen (P< .05), see Table 4 and Figure 3.
Table 4. Comparison of clinical response.
| Response | Group 1 | Group 2 | P value |
|---|---|---|---|
| Complete remission | 5% | 6% | >0.05 |
| Good response | 8% | 22% | < 0.05 |
| Poor response | 42% | 42% | >0.05 |
| No response | 45% | 30% | < 0.05 |
Figure 3. Graphical comparison of clinical response
4. Discussion
We selected this study on 64 OLP patients of either gender which were randomized to received either 0.1% triamcinolone and 5% curcumin. As there is dysregulation of T-cell mediated immunity, blocking the activity of IL-12, TNF-\(\alpha\), IFN-\(\gamma\), MMP-9, RANTES and up-regulating TGF-\(\beta\)1 will benefit the treatment aspects of OLP [11]. No radical therapy for OLP is present, inspite of increased focus on pathogenesis and treatment options [12,13]. Main objective of treatment is to control and reduce the symptoms, eliminate lesions, decrease malignant transformation. Calcineurin inhibitors, retinoids, dapsone, hydroxychloroquine, steroid sparing agents like azathioprine are applied for OLP, still proper treatment regime till now is not available [14]. Systemic/topical corticosteroid is a mainstay drug but has many side-effects such as high blood pressure, adrenal suppression, secondary candidiasis and high recurrence rate. The trend towards the drugs of natural or herbal origin with antioxidant, anti-inflammatory, anti-cancer properties have been considered [15].Our study showed that the mean age was 45.2 years in group 1 and 43.7 years in group 2. There were10 males and 22 females in group 1 and 11 males and 21 females in group 2. Dharman et al., [14] study comprised of 315 OLP patients, wherein four studies revealed that topical curcumin had no statistically significant difference when compared to corticosteroids in treating pain, burning sensation, erythema and ulceration. In three clinical trials compared with placebo, one study showed statistical significance with increased oral dosage (6000 mg) of curcumin, two were not significant due to its reduced oral dosage (2000 mg). Two studies showed that curcumin was effective with increased concentration. Three studies with no controls were statistically significant in reducing burning sensation and clinical appearance of OLP.
Our study demonstrated that pain at baseline (VAS) was 5.48in group 1 and 5.12 in group 2. At baseline, lesion size was 3.85 cm2 in group 1 and 3.72 \(cm^{2}\) in group 2. Keshari et al., [16] assessed the efficacy and safety of topical curcumin in the management of OLP. 27 adult OLP patients, who were randomly divided into two groups. The control group (n = 12) was treated with triamcinolone acetonide 0.1% and the study group (n = 15) with commercially available topical curcumin ointment each to be applied thrice daily for 2 weeks. The patients were reviewed every week. The research groups showed a significant reduction in all the parameters measured. The comparison showed significant improvement in the erythema (P = 0.002), but non-significant reduction in pain (P = 0.697), and ulceration (P = 0.291) in the study group as compared to the control group.
Our findings were common site was buccal mucosa in group 1- 15 and group 2- 17, maxillary gingiva in group 1- 6 and group 2- 5, labial mucosa in group 1- 4, group 2- 6, mandibular gingiva in group 1- 3, group 2- 2, tongue in group 1- 2, group 2- 1, floor of mouth in group 1- 1, group 2- 1 and palate in group 1- 1, group 2- 0.
Thomas AE et al., [17] assessed increased reduction of burning sensation measured by NRS, observed in triamcinolone acetonide group, followed by curcumin 6 times daily application. Curcumin oral gel, applied 3 times a day, was insignificant compared to TA. Application of curcumin oral gel six times daily was considered equally effective compared to triamcinolone acetonide. There was comparable effect in pain reduction score in both triamcinolone and curcumin groups with non- statistical significant difference between two groups. Effects were similar to those of topical corticosteroids, and thus, it can be a suitable alternative to corticosteroids.
Chainani-Wu et al., [18] administered a primary dose of systemic prednisone (60 mg/day for the first week) in both groups, which was not done in our study and could have affected their final results. Long periods of observation not only can interfere with patient compliance and cooperation. Curcumin, a phytochemical has been used as a dietary supplement as well as a therapeutic agent. Curcumin displays a wide range of pharmacological properties against various human disorders. Curcumin exhibits strong anti-inflammatory, analgesic, antioxidant, anti-aging, chemopreventive, antitumoral, anti-angiogenic, anti-metastatic, radio sensitizing, and chemosensitizing effects in cancer. Of therapeutic interest, studies have indicated that curcumin as a lone therapeutic agent is safe and exhibits no major toxicity [19].
5. Conclusion
Oral lichen planus is common inflammatory autoimmune disease. Curcumin in our study showed slightly better results than triamcinolone, hence suggesting that it can be used in patients with oral lichen planus.Author Contributions
All authors contributed equally to the writing of this paper. All authors read and approved the final manuscript.Conflicts of Interest
"The authors declare no conflict of interest.''References
- Manifar, S., Obwaller, A., Gharehgozloo, A., Boorboor Shirazi Kordi, H. R., & Akhondzadeh, S. (2012). Curcumin gel in the treatment of minor aphthous ulcer: A randomized, placebo-controlled trial. Journal of Indian Academy of Oral Medicine and Radiology, 11(41), 40-45. [Google Scholor]
- Das, D. A., Balan, A., & Sreelatha, K. T. (2010). Comparative study of the efficacy of curcumin and turmeric oil as chemopreventive agents in oral submucous fibrosis: A clinical and histopathological evaluation. Journal of Indian Academy of Oral Medicine and Radiology, 22(2), 88-92. [Google Scholor]
- Chainani-Wu, N., Silverman Jr, S., Reingold, A., Bostrom, A., Lozada-Nur, F., & Weintraub, J. (2008). Validation of instruments to measure the symptoms and signs of oral lichen planus. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 105(1), 51-58. [Google Scholor]
- Krupaa, R. J., Sankari, S. L., Masthan, K. M. K., & Rajesh, E. (2015). Oral lichen planus: An overview. Journal of Pharmacy & Bioallied Sciences, 7(Suppl 1), S158-61. [Google Scholor]
- Eisen, D., Ellis, C. N., Duell, E. A., Griffiths, C. E., & Voorhees, J. J. (1990). Effect of topical cyclosporine rinse on oral lichen planus: a double-blind analysis. New England Journal of Medicine, 323(5), 290-294. [Google Scholor]
- Thongprasom, K., Prapinjumrune, C., & Carrozzo, M. (2013). Novel therapies for oral lichen planus. Journal of Oral Pathology & Medicine, 42(10), 721-727. [Google Scholor]
- Irving, G. R., Karmokar, A., Berry, D. P., Brown, K., & Steward, W. P. (2011). Curcumin: the potential for efficacy in gastrointestinal diseases. Best Practice & Research Clinical Gastroenterology, 25(4-5), 519-534. [Google Scholor]
- Thongprasom, K., & Dhanuthai, K. (2008). Steriods in the treatment of lichen planus: a review. Journal of Oral Science, 50(4), 377-385. [Google Scholor]
- Silverman Jr, S., Gorsky, M., & Lozada-Nur, F. (1985). A prospective follow-up study of 570 patients with oral lichen planus: persistence, remission, and malignant association. Oral Surgery, Oral Medicine, Oral Pathology, 60(1), 30-34. [Google Scholor]
- Eisen, D. (2002). The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. Journal of the American Academy of Dermatology, 46(2), 207-214. [Google Scholor]
- Bright, J. J. (2007). Curcumin and autoimmune disease. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease, 595, 425-451. [Google Scholor]
- Nagpal, M., & Sood, S. (2013). Role of curcumin in systemic and oral health: An overview. Journal of Natural Science, Biology, and Medicine, 4(1), 3-7. [Google Scholor]
- Rao, C. V., Simi, B., & Reddy, B. S. (1993). Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis, 14(11), 2219-2225. [Google Scholor]
- Dharman, S., & Ravinthar, K. (2020). Role of curcumin in alleviating symptomatic oral lichen planus: A systematic review. Journal of Clinical & Diagnostic Research, 14(2), ZC134-ZC37. [Google Scholor]
- Kia, S. J., Shirazian, S., Mansourian, A., Fard, L. K., & Ashnagar, S. (2015). Comparative efficacy of topical curcumin and triamcinolone for oral lichen planus: a randomized, controlled clinical trial. Journal of Dentistry (Tehran, Iran), 12(11), 789-796. [Google Scholor]
- Keshari, D., Patil, K., & Mahima, V. G. (2015). Efficacy of topical curcumin in the management of oral lichen planus: A randomized controlled-trial. Journal of Advanced Clinical and Research Insights, 2(5), 197-203. [Google Scholor]
- Thomas, A. E., Varma, B., Kurup, S., Jose, R., Chandy, M. L., Kumar, S. P., ... & Ramadas, A. A. (2017). Evaluation of efficacy of 1% curcuminoids as local application in management of oral lichen planus–interventional study. Journal of Clinical and Diagnostic Research, 11(4), ZC89-ZC93.[Google Scholor]
- Chainani-Wu, N., Silverman Jr, S., Reingold, A., Bostrom, A., Mc Culloch, C., Lozada-Nur, F., & Weintraub, J. (2007). A randomized, placebo-controlled, double-blind clinical trial of curcuminoids in oral lichen planus. Phytomedicine, 14(7-8), 437-446. [Google Scholor]
- Kia, S. J., Basirat, M., & Estakhr, L. (2017). The effect of oral curcumin on pain and clinical appearance of oral lichen planus. Journal of Dentomaxillofacial, 6(1), 1-7. [Google Scholor]