Trends in Clinical and Medical Sciences
ISSN: 2791-0814 (online) 2791-0806 (Print)
DOI: 10.30538/psrp-tmcs2022.0028
Comparative study between oxycodone versus morphine, with zofran, to reduce post-operative nausea and vomiting: a monocentric clinical trial
Joseph Maalouli MD\(^{1,*}\), Patricia Nehme MD\(^{2}\), Marie Merheb MD\(^{3}\) and Elie Gharios MD\(^{4}\)
\(^{1}\) Instructor of Anesthesiology, Mount Lebanon Hospital-Balamand University Medical Center, Lebanon.
\(^{2}\) Instructor of Anesthesiology, Mount Lebanon Hospital-Balamand University Medical Center, Lebanon.
\(^{3}\) Assistant Medical Director for Clinical Affairs at Mount Lebanon Hospital-Balamand University Medical Center, Lebanon.
\(^{4}\) Medical Director at Mount Lebanon Hospital-Balamand University Medical Center, Lebanon.
Correspondence should be addressed to Joseph Maalouli MD at maaloulijo@hotmail.com
Abstract
Subjects and methods: A prospective, monocentric clinical trial study was conducted at Mount Lebanon Hospital (MLH) between November 2018 and November 2021. After getting approval from the ethical committee, 237 patients were enrolled on a ratio of 1 to 2 in two groups: the first receiving Oxycodone 5 mg IVP with Zofran 4 mg IVD and the second receiving Morphine 5 mg IVP with Zofran 4mg IVD. Pearson’s Chi-square test and Fisher’s exact test were used to checking for the groups’ differences.
Results: Morphine and Oxycodone had a similar analgesic effect. The use of Zofran lowered PONV incidence rates in both groups. A statistically significant (p-value = 0.047) lower nausea and vomiting incidence in the Morphine group (2.5%) was noted compared to the Oxycodone group (8.8\% incidence). Only 10% of Oxycodone group patients and 8.9% of Morphine group patients had moderate nausea and vomiting, and none of the group’s patients had severe nausea and vomiting. Surgery duration, gender, age, smoking, BMI, pain level, and treatment group were not statistically associated with the severity of NV.
Conclusion: With the same analgesic effect, Zofran injection seemed to lower PONV incidence in both groups, even though the Morphine group had a lower incidence. Zofran seemed effective in lowering the NV severity as well. Therefore, recommending the systematic administration of antiemetic agents in patients receiving Oxycodone or Morphine could enhance patient satisfaction.
Keywords:
1. Introduction
Worldwide, medical centers try to improve the health care system in its trilogy: care, health, and cost. Patient satisfaction is pointed out as an important clinical indicator for evaluating service quality. However, postoperative nausea and vomiting is still one of the most common and unpleasant side effects following surgeries under general anesthesia, with a mean overall incidence between 30-40% [1].
Perioperative opioid use remains one of the major participants in this issue. Opioids have been the mainstay of pain management for thousands of years, owing to their cost effectiveness in both the inpatient and outpatient settings. Aside from these attributes, opioids have significant side effects associated with nausea, vomiting, constipation, physical dependence, tolerance, and respiratory depression [2].
Morphine, the prototype opiate agent, traces its origin to single plant-the opium poppy. It has been used for centuries for recreation before it became widely used as a pain reliever, particularly in the 1800s. Due to its long analgesic duration of 4-5 hours, low cost, and extensive availability, it is often selected to control postoperative pain [3] preemptively.
Nevertheless, the disadvantages of morphine: slow onset of analgesia (20 min), unsuitability for hemodynamically unstable patients, and several other adverse effects have shed light on recent studies that adopt the use of alternative opioids or combination therapy to tackle this problem [4].
On the other hand, Oxycodone, a semi-synthetic opioid alkaloid derived from Thebaine, is a moderately potent opioid analgesic, developed in 1916 in Germany and has recently been used in Lebanon in an attempt to improve the existing opioids [5].
The recent interest in Oxycodone is based on its favorable pharmacokinetics and pharmacodynamics, especially in the central nervous system [6]. Moreover, relatively high enteral bioavailability allows an easy switch from one drug formulation to another during the course of pain management.
Oxycodone is highly effective and well tolerated in different types of surgical procedures and patient groups. One study conducted at New York University Langone Medical Center in the United States of America showed that the incidence of postoperative nausea and vomiting associated with Oxycodone is 19% [7], which is believed to surpass all the previous opioids, and mainly morphine.
Antiemetic agents were added to reduce one of the well-known side effects of opioids in postoperative settings. Zofran (Ondansetron) is a selective 5-HT3 receptor antagonist, approved by the FDA in 1991, triggers the area postrema in the brainstem [8], with primary effects in the gastrointestinal tract, reducing, therefore, nausea and vomiting within 30 min after IV or IM administration.
Our study aims to add to the previous research that aimed to find the best remedy for postoperative nausea and vomiting to improve patient satisfaction. Alongside pain, PONV is one of the most frequently encountered problems after surgical procedures, and its incidence could reach 30% [1]. While pain management is currently well managed, as well as morbidity and mortality from anesthetic agents, patients' satisfaction is affected by their PONV experience [7]. Patients were sometimes willing to sacrifice by bearing pain, so they would not experience NV [4]. PONV could also affect patients' bills by increasing their length of stay at the PACU. Therefore, recommendations to prevent and manage PONV are currently gaining interest, and research in this field is growing. Nausea and vomiting could be perceived as normal reactions of the body to any emetic stimulus. This includes a variety of stimuli such as food toxins and side effects of drug therapy. The stimulus is first detected by the abdominal vagal afferents, the area posterma, and the vestibular system. This leads to nausea, an unpleasant but nonpainful reaction, which prepares the body to learn either aversion or avoidance. Another reaction to this stimulus detection (that could also be present with it) is vomiting, a reflex reaction that helps to eject the toxins from the gastrointestinal tract. Studies showed that surgery duration, gender, age, smoking, BMI, pain level, and treatment agent were associated with a higher incidence of PONV [1]. A systematic review of the literature summarized evidence levels for all risk factors of PONV. The risk factors are mainly anesthesia dependents, such as volatile anesthetics and nitrous oxide administration, or concern the postoperative and intraoperative administration of opioids.
Prevention of PONV relies first on avoiding its risk factors where possible and preferring less emetic anesthetic agents and pain killers whenever possible. Zofran (Ondansetron) is a selective 5-HT3 receptor antagonist, approved by the FDA in 1991, triggers the area postrema in the brainstem [8], with primary effects in the gastrointestinal tract, reducing, therefore, nausea and vomiting within 30 min after IV administration. Moreover, its administration was known to reduce the incidence of PONV in adults [9] and pediatric population [10]. Besides, the Ondansetron effect with a 4mg dosage was found to be superior to 10 mg of metoclopramide in preventing PONV incidence and giving higher patient satisfaction scores in a systematic review of literature [11].
2. Objectives
2.1. Primary objective
The primary purpose of this study was to compare which combination Zofran and Oxycodone versus Zofran and Morphine is better for the prevention of post-operative nausea and vomiting and improvement of patient satisfaction.2.2. Secondary objectives
Our study's secondary objectives were to:- Evaluate the safety, acceptability and effectiveness of Zofran in decreasing post- operative nausea and vomiting.
- Detect the factors that affect the nausea and vomiting severity in our target population.
3. Subjects and methods
3.1. Ethical consideration
First of all, the clearance and approval of the ethical committee at Mount Lebanon Hospital was obtained on November 13, 2018 to be able to start recruitment. All patients willingly participated to the study after signing the informed consent before their surgery. They were informed by the study team about the study, that they will be blinded to the treatment they receive, as well as about their right to drop out from the study at any time. Besides, all data was confidential, patients' identities were to remain only available for their doctors and the treating team during their hospitalization, and no use of any personal information was intended for any purpose other than this research.3.2. Study design
A prospective, monocentric clinical trial study was conducted at Mont Lebanon Hospital (MLH), between November 2018 and November 2021, to assess the post-operative nausea and vomiting PONV in patients undergoing elective surgeries under general anaesthesia. It was double blinded: study participants and the anesthesiologists collecting and analyzing clinical data were unaware of the assigned treatment. After obtaining written informed consent, the patients were divided into two groups, each receiving a treatment. Patients were thus enrolled on a ratio of 1 to 2 in two groups: the first receiving Oxycodone 5 mg IVP with Zofran 4 mg IVD and the second receiving Morphine 5 mg IVP with Zofran 4mg IVD.3.3. Study population
Our study population consisted of 237 patients who underwent elective surgeries and who had a numerical rating scale (NRS) of 6 /10 or more at the post anesthetic care unit (PACU).3.4. Inclusion criteria
Subjects must meet the following criteria to be enrolled in the trial:- Male or female aged between 19 and 65 years.
- Fits the American Society of Anesthesiology ASA1 and ASA2
- Undergoing elective surgery under general anesthesia
3.5 Exclusion criteria
Subjects are excluded from enrolment if any of the following criteria is present:- Age below 18 or more than 65 years
- Known allergy to opioid or ondansetron
- Known Chronic obstructive pulmonary disease (COPD)
- Known to have moderate to severe hepatic failure
- Known to have Severe renal insufficiency (serum creatinine >1.6 mg/dl)
- Current Pregnancy
- Patient with head injury
- Antiemetic use within 24 hours
3.6. Sample size
Recent studies showed that the incidence of post-operative nausea and vomiting after oxycodone use post general anesthesia was 19% [7] versus 12%-38% for morphine [12]. A 50% decrease in the incidence down to 9% with Zofran was considered clinically significant with alpha=0.05 and beta = 0.2. An intermediate analysis was performed, with a total sample of 237 patients, divided into 2 groups, in whom the informed consent was obtained, and who fitted the eligibility criteria.4. Material and procedure of data collection
4.1. Material
Zofran 4mg IVD was given systematically to all patients 30 min before the end of the surgery. Oxycodone 5 mg IVD was given in the PACU in the first group of patients. For the second one, Morphine 5 mg IVD was used.4.2. Procedure
Before induction of anesthesia, all patients were assessed , description of the treatment plan as well as exciting alternatives were explained , and participation in the study was obtained through informed consent . Thirty minutes before the end of the operation, both groups were given 4mg Zofran. All patients were then escorted to the post anesthesia care unit (PACU) and monitoring was standardized for all patients including ECG, pulse oximetry and noninvasive blood pressure (NIBP). Those who had a pain scale more than 6 /10, received morphine or oxycodone and were monitored for the next 2 hours. A questionnaire was filled by the anesthesiologists to assess post-operative nausea and vomiting after oxycodone use vs. morphine, each with the same dose of Zofran 4 mg. All episodes of nausea and emesis were recorded using a severity scale from 0 to 2 under the assessment time frame from 0 to 2 hours:- T0: PONV immediately after receiving pain killer.
- T1: PONV at 1 hour after receiving pain killer.
- T2: PONV at 2 hours after receiving pain killer.
5. Data analysis
Data entry was done on Excel. Data analysis was done using SPSS version 22. Categorical variables were represented using frequencies and percentages. Pearson's chi square test was used to check the difference between groups for categorical variables. Fisher's Exact test was used instead of it when the cell count wasn't enough to perform Pearson's chi square test. First groups differences were assessed, then the variables affecting the nausea and vomiting (NV) severity were evaluated. Finally, nausea and vomiting incidence at T0, T1, and T2 were evaluated.6. Results
6.1. Nausea and vomiting incidence in groups
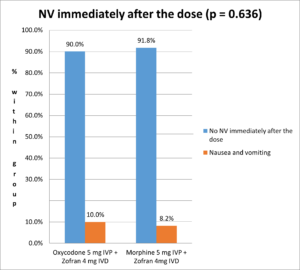
The incidence of nausea and vomiting immediately after the dose is 10% in Oxycodone group and 8.2% in Morphine group, without any statistically significant difference between the two treatment groups (Fisher's Exact Test p-value = 0.636) (Figure 1).
Figure 1. Nausea and vomiting incidence immediately after the dose.
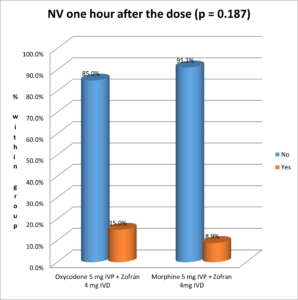
The following figure shows the incidence of nausea and vomiting one hour after the dose: 15% of Oxycodone patients experienced NV, versus 8.9 % in the group who received Morphine, without any statistically significant difference (Pearson's chi square test p-value = 0.187) (Figure 2).
Figure 2. Nausea and vomiting incidence one hour after the dose.
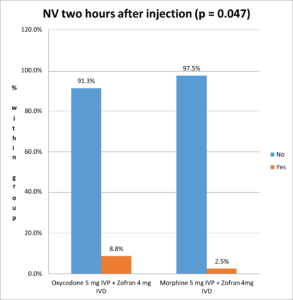
There was a statistically significant (Pearson's chi square test p-value = 0.047 < 0.05) lower nausea and vomiting incidence in the Morphine group (2.5%) compared to the Oxycodone group (8.8% incidence) (Figure 3).
Figure 3. Nausea and vomiting incidence two hours after injection.
7. Discussion
This monocentric controlled trial was mainly conducted to compare which combination Zofran and oxycodone versus Zofran and morphine is better for the prevention of post-operative nausea and vomiting and improvement of patient satisfaction. It also aimed to evaluate the safety, acceptability and effectiveness of Zofran in decreasing post-operative nausea and vomiting, and to detect the factors that affect the nausea and vomiting severity in our target population. 237 patients were divided into two groups. We assessed the analgesic effects of both treatments by checking the differences in NRS scale. We evaluated Zofran efficacy in reducing the incidence and the severity of PONV. Finally, we evaluated the factors associated with severity of PONV.Our study showed a statistically significant (p-value = 0.047) lower nausea and vomiting incidence in the Morphine group (2.5%) compared to the Oxycodone group (8.8% incidence). These results match those found in 2013 by Pedersen and his colleagues, who concluded that Morphine had significantly less nausea than Oxycodone (p = 0.03) [13]. However, another study leaded by Lenz in 2009 found no difference between the two drugs in PONV incidence [14]. On the other hand, Oxycodone showed less secondary effects including sedation, when compared to Morphine and other opioids like Fentanyl and Sufentanyl [15]. Therefore, the controversy found in literature highlights the need for solid meta-analysis to get conclusive results about the safety and tolerance of Oxycodone and Morphine in postoperative pain management.
Moreover, our study aimed to assess the efficacy of Zofran for PONV prevention. Recent studies showed that the incidence of post-operative nausea and vomiting after oxycodone use post general anesthesia was 19% [7] vs 12% to 38% for morphine [12]. However, our results showed lower incidence rates of PONV: we found an 8.8% incidence in the Oxycodone group and a 2.5% incidence rate in the Morphine group. Therefore, the use of Zofran injection reduced by almost 2.16 times the incidence of NV in Oxycodone group, and by 4.8 to 15.2 times in the Morphine group. A 50% reduction of NV was considered clinically relevant by the researchers so the results obtained seemed way beyond expected and effectively very suitable for the patients.
Regarding PONV severity, our results showed that only 10% of Oxycodone group patients and 8.9% of Morphine group patients had moderate nausea and vomiting, and none of the groups' patients had severe nausea and vomiting. To our knowledge, no other studies in the literature assessed the severity of NV in patients of our target population. However, since none of the patients reported severe nausea, then it seems that Zofran 4mg injection was effective in lowering the NV severity in the recruited patients (besides reducing its incidence).
Our study showed that neither surgery duration, nor gender, nor age, nor smoking, nor BMI, nor NRS pain level, nor treatment group were statistically associated with severity of nausea and vomiting. However, all the before mentioned were reported in other studies as risk factors of PONV [1,16]. The fact that none of these was detected as aggravating factors of PONV could be explained by the design of our study that included only a certain group of patients where all of them were with high pain levels, all of them receiving opioid injections, all being selected within a predefined age rank, which makes the homogeneity between the patients higher.
7.1. Study strengths
This study is one of few comparing Oxycodone to Morphine, both with Zofran injection and the first in Lebanon, particularly to our knowledge. Besides, this controlled trial was done without a massive budget, with the available standard of care surveillance, comparing frequently used drugs on a daily basis, so no funding was obtained to perform it, and it relied on the devotion of the researchers mainly. Nevertheless, patients were under the best safety measures, with the best surveillance methods in PACU since MLH is a university hospital well equipped for that purpose.7.2. Study limitations and perspectives
Despite all the strengths, this study had some limitations worth mentioning. Other factors affecting the PONV incidence, such as delayed gastric emptiness, and anesthetic agent used during surgery, were not taken into account in this study. Therefore, it would be interesting to include them in future studies. Finally, only PONV was measured among all opioid side effects, so the evaluation of safety was bound only to PONV in this study. Future studies should consider evaluating the difference between Oxycodone and Morphine with all side effects accounted for in the design.The study was conducted to find the best remedy to prevent PONV, one of the most common and unpleasant side effects following surgeries. Patients were sometimes willing to sacrifice by bearing pain so they would not experience NV [4]. Based on our results, measures to improve patient outcomes, public health, and health services should be taken, such as recommending the systematic administration of antiemetic agents to prevent or reduce the severity of PONV in patients receiving Oxycodone or Morphine.
8. Conclusion
This monocentric clinical trial was the first in Lebanon to assess the difference between Oxycodone and Morphine coupled with Zofran in reducing PONV incidence. It also aimed to evaluate Zofran's safety, acceptability, and effectiveness in decreasing postoperative nausea and vomiting and to detect the factors affecting the severity of nausea and vomiting in our target population. Two hundred thirty-seven patients were divided into two groups, each receiving a treatment. We assessed the analgesic effects of both treatments by checking the differences on the NRS scale. We evaluated the efficacy of the treatment by assessing the PONV incidence difference. Zofran efficacy was evaluated in reducing the incidence and the severity of PONV. The factors associated with the severity of PONV were also assessed.Regarding analgesic effects, our results showed that Morphine and Oxycodone seemed to have similar analgesic effects. Our results also showed clinically relevant lower incidence rates of PONV in both Oxycodone and Morphine groups. Moreover, Zofran injection seemed effective in lowering the NV severity. Neither surgery duration, gender, age, smoking, BMI, nor NRS pain level, nor treatment group was statistically associated with the severity of nausea and vomiting.
The study was conducted to find the best remedy to prevent PONV, one of the most common and unpleasant side effects following surgeries. Based on our results, recommending the systematic administration of antiemetic agents to prevent or reduce the severity of PONV in patients receiving Oxycodone or Morphine could enhance patients' satisfaction. Future studies could focus on including all the factors affecting PONV incidence and evaluate all their side effects to accurately estimate the efficacy and safety of Zofran as an antiemetic.
Author Contributions:
All authors contributed equally to the writing of this paper. All authors read and approved the final manuscript.Conflicts of Interest:
The authors declare no conflict of interest.References
- Rüsch, D., Eberhart, L. H., Wallenborn, J., & Kranke, P. (2010). Nausea and vomiting after surgery under general anesthesia: an evidence-based review concerning risk assessment, prevention, and treatment. Deutsches Ärzteblatt International, 107(42), 733-741. [Google Scholor]
- Benyamin, R., Trescot, A. M., Datta, S., Buenaventura, R. M., Adlaka, R., Sehgal, N., ... & Vallejo, R. (2008). Opioid complications and side effects. Pain Physician, 11(2S), S105-120. [Google Scholor]
- Zirpe, K., & Bamne, S. N. (2020). Opiate and cerebral atrophy. Indian Journal of Critical Care Medicine: Peer-reviewed, Official Publication of Indian Society of Critical Care Medicine, 24(4), 218-219. [Google Scholor]
- Gregorian Jr, R. S., Gasik, A., Kwong, W. J., Voeller, S., & Kavanagh, S. (2010). Importance of side effects in opioid treatment: a trade-off analysis with patients and physicians. The Journal of Pain, 11(11), 1095-1108. [Google Scholor]
- Kalso, E. (2005). Oxycodone. Journal of Pain and Symptom Management, 29(5), 47-56. [Google Scholor]
- E B. Pharmacokinetics and pharmacodynamics of oxycodone and morphine with emphasis on blood-brain barrier transport. 2011 Jun 13 [cited 2020 Nov 20]; Available from: https://europepmc.org/article/eth/8591. [Google Scholor]
- Cavalcanti, I. L., Carvalho, A. C. G. D., Musauer, M. G., Rodrigues, V. S., Migon, R. N., Figueiredo, N. V., & Vane, L. A. (2014). Safety and tolerability of controlled-release oxycodone on postoperative pain in patients submitted to the oncologic head and neck surgery. Revista do Colégio Brasileiro de Cirurgiões, 41, 393-399. [Google Scholor]
- Gan, T. J. (2005). Selective serotonin 5-HT3 receptor antagonists for postoperative nausea and vomiting. CNS Drugs, 19(3), 225-238. [Google Scholor]
- Zhang, D., Shen, Z., You, J., Zhu, X., & Tang, Q. F. (2013). Effect of ondansetron in preventing postoperative nausea and vomiting under different conditions of general anesthesia: A preliminary, randomized, controlled study. Upsala Journal of Medical Sciences, 118(2), 87-90. [Google Scholor]
- Ummenhofer, W., Frei, F. J., Urwyler, A., Kern, C., & Drewe, J. (1994). Effects of ondansetron in the prevention of postoperative nausea and vomiting in children. Anesthesiology, 81(4), 804-810. [Google Scholor]
- Cox, F. (1999). Systematic review of ondansetron for the prevention and treatment of postoperative nausea and vomiting in adults. British Journal of Theatre Nursing (United Kingdom), 9(12), 556-566. [Google Scholor]
- Chandrakantan, A., & Glass, P. S. A. (2011). Multimodal therapies for postoperative nausea and vomiting, and pain. British Journal of Anaesthesia, 107(suppl1), i27-i40. [Google Scholor]
- Pedersen, K. V., Olesen, A. E., Drewes, A. M., & Osther, P. J. S. (2013). Morphine versus oxycodone analgesia after percutaneous kidney stone surgery. Urolithiasis, 41(5), 423-430. [Google Scholor]
- Lenz, H., Sandvik, L., Qvigstad, E., Bjerkelund, C. E., & Raeder, J. (2009). A comparison of intravenous oxycodone and intravenous morphine in patient-controlled postoperative analgesia after laparoscopic hysterectomy. Anesthesia & Analgesia, 109(4), 1279-1283. [Google Scholor]
- Raff, M., Belbachir, A., El-Tallawy, S., Ho, K. Y., Nagtalon, E., Salti, A., ... & Hadjiat, Y. (2019). Intravenous oxycodone versus other intravenous strong opioids for acute postoperative pain control: a systematic review of randomized controlled trials. Pain and Therapy, 8(1), 19-39. [Google Scholor]
- Shaikh, S. I., Nagarekha, D., Hegade, G., & Marutheesh, M. (2016). Postoperative nausea and vomiting: A simple yet complex problem. Anesthesia, Essays and Researches, 10(3), 388-396. [Google Scholor]