Trends in Clinical and Medical Sciences
ISSN: 2791-0814 (online) 2791-0806 (Print)
DOI: 10.30538/psrp-tmcs2022.0027
Histopathological patterns in the endometrial biopsy of patients presenting with abnormal uterine bleeding
Akbarova Munisa Abduxalilovna\(^{1}\) and Sarvar Akbarov Alisherovich\(^{2,*}\)
\(^{1}\) Faculty of Medical and Pedagogical Affairs, Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan.
\(^{2}\) Director of Private Medical Clinic Med Elite, Center for the Development of professional Qualifications of Medical workers, Tashkent, Uzbekistan.
Correspondence should be addressed to Sarvar Akbarov Alisherovich at urolog.sarvar@gmail.com
Abstract
Methodology: One hundred eight females with the complaint of abnormal uterine bleeding were enrolled. A gynecological examination was done. Dilatation and curettage were carried out. Specimens thus obtained were stored in 10\% formalin. The slides were examined under a microscope, and the various histopathological patterns were assessed.
Results: The age group 20-30 years had 48, 30-40 years had 50, and 40-50 years had ten females. A significant difference was observed. Inflammatory lesions were 28, such as acute endometritis in 12, chronic endometritis in 11, and chorioamnionitis in 5. Proliferative non-neoplastic lesions were 42, such as atypical hyperplasia in 18, typical hyperplasia in 12, and endometrial polyp in 12. Neoplastic lesions in 20 include leiomyoma in 8, a partial mole in 7, the complete mole in 2, and endometroid carcinoma in 3. Normal endometrium in 18, such as proliferative phase in 12 and secretory phase endometrium in 6. A significant difference was observed (P< 0.05).
Conclusion: The most common endometrial biopsy revealed proliferative non-neoplastic lesions such as atypical hyperplasia, typical hyperplasia, and endometrial polyp.
Keywords:
1. Introduction
Abnormal uterine bleeding (AUB) is a commonly occurring gynecological complaint characterized by abnormal blood loss, duration of flow and frequency of menstruation [1]. It is evident among 30-35% of all females. The major outcome of AUB is anemia, which greatly impacts females' health quality [2]. Among various causes of abnormal uterine bleeding, dysfunctional menometrorrhagia is a common one. To reach the diagnosis of AUB, a careful history as well as physical examination is required [3]. It is a challenging task among gynecologists. Transvaginal ultrasound may be helpful in up to 60% of cases, and the cause of the bleeding is recognized in only 50-60% of the cases. There can be various causes of AUB, such as physiological, pathological, or pharmacological [4,5].
It has been found to be linked with almost any type of endometrium, ranging from normal endometrium to hyperplasia, irregular ripening, chronic menstrual irregular shedding, and atrophy [6]. Histological variations of the endometrium are useful in detecting various disease patterns. It can be assessed with the help of the age of patients, the phase of the menstrual cycle, and iatrogenic use of hormones[7].
Histological variations of the endometrium can be detected, considering the woman's age, the phase of her menstrual cycle, and iatrogenic use of hormones [8]. It is found that in about 10% of patients, endometrial cancer may be the outcome of abnormal perimenopausal or postmenopausal bleeding [9]. Atypical endometrial hyperplasia is the outcome of endometrial cancer and may progress over time to endometrial cancer in 5-25% of patients[10]. Considering this, we attempted present a study to assess histopathological patterns in the endometrial biopsy of patients presenting with abnormal uterine bleeding.
2. Methodology
A total of one hundred eight females with the complaint of abnormal uterine bleeding was enrolled. Inclusion criteria was females within age group 20-50 years and those giving written consent was enrolled. Those did not wish to participate were excluded from the study. The approval for the study protocol was obtained from institutional ethical clearance committee. All enrolled patients gave written consent for the study.Demographic data of each patient was recorded. A detailed history of each patient was recorded. A thorough physical examination was performed. Pelvic ultrasound was performed. Dilatation and curettage were carried out. Specimens thus obtained were stored n 10% formalin. A gross study was done and multiple sections were obtained. The specimens were processed in automated tissue processor. Four to six-micron thick paraffin embedded sections were taken and stained by haematoxylin and eosin. The slides were examined under microscope and the various histopathological patterns assessed. Statistical assessment was carried using MS excel sheet with SPSS version 20.0. The level of significance was set below 0.05.
3. Results
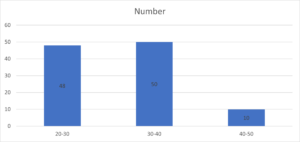
Age group 20-30 years had 48, 30-40 years had 50 and 40-50 years had 10 females. A significant difference was observed (P< 0.05) (Table 1, Figure 1).
Table 1. Age wise distribution.
| Age group (years) | Number | P value |
|---|---|---|
| 20-30 | 48 | 0.05 |
| 30-40 | 50 | |
| 40-50 | 10 |
Figure 1. Age wise distribution.
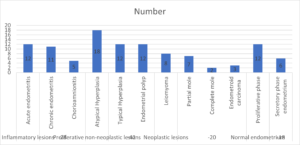
Inflammatory lesions were 28, such as acute endometritis in 12, chronic endometritis in 11, and chorioamnionitis in 5. Proliferative non-neoplastic lesions were 42, such as atypical hyperplasia in 18, typical hyperplasia in 12, and endometrial polyp in 12. Neoplastic lesions in 20 include leiomyoma in 8, a partial mole in 7, a complete mole in 2, and endometroid carcinoma in 3.
Normal endometrium in 18, such as proliferative phase in 12 and secretory phase endometrium in 6. A significant difference was observed (P< 0.05) (Table 2, Figure 2).
Inflammatory lesions were 28, such as acute endometritis in 12, chronic endometritis in 11, and chorioamnionitis in 5. Proliferative non-neoplastic lesions were 42, such as atypical hyperplasia in 18, typical hyperplasia in 12, and endometrial polyp in 12. Neoplastic lesions in 20 include leiomyoma in 8, a partial mole in 7, a complete mole in 2, and endometroid carcinoma in 3.
Normal endometrium in 18, such as proliferative phase in 12 and secretory phase endometrium in 6. A significant difference was observed (P< 0.05) (Table 2, Figure 2).
Figure 2. Histopathological diagnosis of endometrial biopsy.
Table 2. Histopathological diagnosis of endometrial biopsy.
| Parameters | Variables | Number |
P value |
|---|---|---|---|
| Inflammatory lesions (28) | Acute endometritis | 12 | <0.05 |
| Chronic endometritis | 11 | ||
| Chorioamnionitis | 5 | ||
| Proliferative non-neoplastic lesions (42) | Atypical Hyperplasia | 18 | >0.05 |
| Typical Hyperplasia | 12 | ||
| Endometrial polyp | 12 | ||
| Neoplastic lesions (20) | Leiomyoma | 8 | <0.05 |
| Partial mole | 7 | ||
| Complete mole | 2 | ||
| Endometroid carcinoma | 3 | ||
| Normal endometrium (18) | Proliferative phase | 12 | >0.05 |
| Secretory phase endometrium | 6 |
4. Discussion
Abnormal uterine bleeding, incomplete abortions, and suspected neoplasia are among a few reasons for taking endometrial biopsies [11,12,13]. It may be sampled previously for specific measures to treat infertility to assess the phase of the cycle to direct extra tests or treatments [14,15,16]. The clinical indication shows the protocol to handle any endometrial sampling material for the specimen submission, which may be for evaluation of infertility or preparation for in vitro fertilization (IVF), evaluation of abnormal uterine bleeding, and follow-up of a previous cytological or histological diagnosis. The endometrium may be examined as part of a hysterectomy specimen and may be the site of a primary or secondary neoplastic process [17,18]. The present study assessed histopathological patterns in the endometrial biopsy of patients presenting with abnormal uterine bleeding.Our study showed that the age group 20-30 years had 48, 30-40 years had 50, and 40-50 years had ten females. Isuzu et al., [19] included 304 cases. Most of the cases of endometrial hyperplasia were typical. Endometritis and chorioamnionitis were the inflammatory conditions seen. It was seen that 23 females had molar pregnancies. The most common cause of abnormal uterine bleeding was retained products of conception.
We observed that inflammatory lesions were 28, such as acute endometritis in 12, chronic endometritis in 11, and chorioamnionitis in 5. Proliferative non-neoplastic lesions were 42, such as atypical hyperplasia in 18, typical hyperplasia in 12, and endometrial polyp in 12. Neoplastic lesions in 20 such as leiomyoma in 8, a partial mole in 7, a complete mole in 2, and endometroid carcinoma in 3. Normal endometrium in 18, such as proliferative phase in 12 and secretory phase endometrium in 6. Doraiswami et al., [20] found that 41-50 years was the most commonly involved age group with abnormal uterine bleeding seen in 33.5%. The most familiar pattern in these patients was normal cycling endometrium seen among 28.4%. The most familiar pathology was a disordered proliferative pattern seen in 20.5%. Other causes identified were complications of pregnancy (22.7%), benign endometrial polyp (11.2%), endometrial hyperplasias (6.1%), carcinomas (4.4%), and chronic endometritis (4.2%). Endometrial causes of AUB and age patterns were statistically significant.
Gunaken et al., [21] in their study, a total of 188 patients were included. The most common histopathological results were endometrial polyp was seen at 26.6%, atrophic endometrium at 22.3%, and surface epithelium at 12.8%. None of the 57 patients without vaginal bleeding had endometrial cancer. In 131 patients with vaginal bleeding, the mean endometrial thickness was 9.8 mm, and the rate of endometrial disorders was 56.5% (74 patients). Endometrial cancer was diagnosed in 19 patients (10.1%), and 36.8% of them had non-endometrioid cancers. The presence of vaginal bleeding was significantly associated with the diagnosis of endometrial cancer and any endometrial disorder.
5. Conclusion
Most common endometrial biopsy revealed proliferative non-neoplastic lesions such as atypical hyperplasia, typical hyperplasia and endometrial polyp.Author Contributions:
All authors contributed equally to the writing of this paper. All authors read and approved the final manuscript.Conflicts of Interest:
''The authors declare no conflicts of interest.''References
- Goldstein, R. B., Bree, R. L., Benson, C. B., Benacerraf, B. R., Bloss, J. D., Carlos, R., ... & Walker, J. (2001). Evaluation of the woman with postmenopausal bleeding: Society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. Journal of Ultrasound in Medicine, 20(10), 1025-1036. [Google Scholor]
- Kamel, H. S., Darwish, A. M., & Mohamed, S. A. R. (2000). Comparison of transvaginal ultrasonography and vaginal sonohysterography in the detection of endometrial polyps. Acta Obstetricia et Gynecologica Scandinavica, 79(1), 60-64. [Google Scholor]
- Timmermans, A., Opmeer, B. C., Khan, K. S., Bachmann, L. M., Epstein, E., Clark, T. J., ... & Mol, B. W. (2010). Endometrial thickness measurement for detecting endometrial cancer in women with postmenopausal bleeding: a systematic review and meta-analysis. Obstetrics & Gynecology, 116(1), 160-167. [Google Scholor]
- Smith-Bindman, R., Weiss, E., & Feldstein, V. (2004). How thick is too thick? When endometrial thickness should prompt biopsy in postmenopausal women without vaginal bleeding. Ultrasound in Obstetrics and Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology, 24(5), 558-565. [Google Scholor]
- Debby, A., Malinger, G., Glezerman, M., & Golan, A. (2006). Intra-uterine fluid collection in postmenopuasal women with cervical stenosis. Maturitas, 55(4), 334-337. [Google Scholor]
- Epstein, E., Ramirez, A., Skoog, L., & Valentin, L. (2001). Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstetricia et Gynecologica Scandinavica, 80(12), 1131-1136. [Google Scholor]
- Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., ... & Bray, F. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer, 136(5), E359-E386. [Google Scholor]
- Kurman, R. J., & Scully, R. E. (1976). Clear cell carcinoma of the endometrium. An analysis of 21 cases. Cancer, 37(2), 872-882. [Google Scholor]
- Zorn, K. K., Bonome, T., Gangi, L., Chandramouli, G. V., Awtrey, C. S., Gardner, G. J., ... & Birrer, M. J. (2005). Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clinical Cancer Research, 11(18), 6422-6430. [Google Scholor]
- Kilbourn, C. L., & Richards, C. S. (2001). Abnormal uterine bleeding: diagnostic considerations, management options. Postgraduate Medicine, 109(1), 137-150. [Google Scholor]
- Yusuf, N. W. (1996). Dysfunctional uterine bleeding: a retrospective clinicomorphological study over two years. Pakistan Journal of Obstetrics and Gynaecology, 9(1), 27-30. [Google Scholor]
- Muzaffar, M., Akhtar, K. A. K., Yasmin, S., Rehman, M., Iqbal, W., & Khan, M. A. (2005). Menstrual irregularities with excessive blood loss: a clinico-pathological correlation. Journal-Pakistan Medical Association, 55(11), 486. [Google Scholor]
- Silverberg, S. G. (2000). Problems in the differential diagnosis of endometrial hyperplasia and carcinoma. Modern Pathology, 13(3), 309-327. [Google Scholor]
- Cho, N. H., Park, C. I., & Choi, I. J. (1989). Clinicopathologic Study of the Endometrium of Dysfunctional Uterine Bleeding. The Korean Journal of Pathology, 23(1), 65-74. [Google Scholor]
- Gredmark, T., Kvint, S., Havel, G., & Mattsson, L. (1995). Histopathological findings in women with postmenopausal bleeding. BJOG: An International Journal of Obstetrics & Gynaecology, 102(2), 133-136. [Google Scholor]
- Hileeto, D., Fadare, O., Martel, M., & Zheng, W. (2005). Age dependent association of endometrial polyps with increased risk of cancer involvement. World Journal of Surgical Oncology, 3(1), 1-6. [Google Scholor]
- Escoffery, C. T., Blake, G. O., & Sargeant, L. A. (2002). Histopathological findings in women with postmenopausal bleeding in Jamaica. The West Indian Medical Journal, 51(4), 232-235. [Google Scholor]
- Dangal, G. (2003). A study of endometrium of patients with abnormal uterine bleeding at Chitwan Valley. Kathmandu University Medical Journal, 1(2), 110-112. [Google Scholor]
- Asuzu, I. M., & Olaofe, O. O. (2018). Histological pattern of endometrial biopsies in women with abnormal uterine bleeding in a hospital in north central nigeria. International Journal of Reproductive Medicine, 2018 Article ID, 2765927. https://doi.org/10.1155/2018/2765927. [Google Scholor]
- Doraiswami, S., Johnson, T., Rao, S., Rajkumar, A., Vijayaraghavan, J., & Panicker, V. K. (2011). Study of endometrial pathology in abnormal uterine bleeding. The Journal of Obstetrics and Gynecology of India, 61(4), 426-430. [Google Scholor]
- Günakan, E., Atak, Z., Albayrak, M., Kurban, Y., & Simsek, G. G. (2018). Endometrial histopathology results and evaluation of endometrial cancer risk in geriatric women. Menopause Review/Przeglad Menopauzalny, 17(1), 18-21. [Google Scholor]