Trends in Clinical and Medical Sciences
ISSN: 2791-0814 (online) 2791-0806 (Print)
DOI: 10.30538/psrp-tmcs2022.0032
Ultrasound guided anterior approach coeliac plexus ethanol neurolysis for pain relief in gastric and pancreatic malignancy
D. Ashok Kumar\(^{1}\), Ganesh Annamalai\(^{2}\), Nadar Kalaivani Venkatasami\(^{2}\) and Sivabalan R G\(^{3,*}\)
\(^{1}\) Department of Anaesthesia and Pain, Tamilnadu Government Multi Super Speciality Hospital, Omandurar estate, Omandurar, Chennai, Tamil Nadu, India.
\(^{2}\) Institute of Anaesthesiology & Critical Care Rajiv Gandhi Government general Hospital, Madras Medical College, Madras, India.
\(^{3}\) Department of Anaesthesia, Institute of Anaesthesiology and Critical Care, Madras Medical College, Chennai, Tamil Nadu, India.
Correspondence should be addressed to Sivabalan R G at sivabalanrg04@rediffmail.com
Abstract
Methods and materials: This is an uncontrolled before-and-after non randomized trial. This bedside procedure was done in an ICU in chronic pain management centre. Trial coeliac plexus block was done after identification of coeliac trunk using ultrasound and 15 cc of 1% xylocard was given. After 30 minutes, pain relief was assessed with Numerical Rating Scale for pain (NRS). Patient was then given 20ml of 60% ethanol. Pain relief was documented using NRS immediately at 24hours, 72 hours,1 week and 3 months. Statistical analysis was done using Statistical Package for the Social Sciences version (SPSS) version 16.0. Paired t test was used to analyze the difference between variables.
Results: Our study shows statistically significant difference between pre-NRS and post immediate-NRS (mean 8.26\(\pm\)0.52 and 3.40 \(\pm\)0.47)(P value \(<0.0001\)) and significant decrease in NRS seen after 24 hours,72 hours,1 week and 3 months.
Conclusion: USG guided anterior approach coeliac plexus ethanol neurolysis is effective in decreasing pain associated with gastric and pancreatic malignancy.
Keywords:
1. Introduction
The incidence of Gastric malignancy is relatively high in southern parts of India [1] particularly in Chennai and the incidence of pancreatic malignancy is also rising in India. Although surgical resection procedures can be done,only 15-20% present themselves to the hospital at that stage. Pain associated with gastric and pancreatic malignancy presents mostly as abdominal pain that often radiates to the back. Mid epigastric Pain is the most common symptom in these patients. About 80% of patients with advanced pancreatic cancer complain of abdominal and/or back pain [2]. At the time of diagnosis, the disease is often advanced and up to 73% of patients suffer from abdominal pain [3]. Inadequately treated pain can have profound negative effects on the psychosocial and physical well being of the patients and it subjects them to an avoidable anxiety and depression. Morbidity and mortality increase in patients with chronic pain. A good pain relief obtained with Coeliac Plexus Neurolysis (CPN) can improve the morbidity, mortality and well being of the patient [4]. Coeliac plexus neurolysis is conventionally done under CT, fluoroscopic guidance or endoscopic ultrasound. Most patients come for pain management in more advanced stages of cancer and they have difficulty in lying in prone position or subjecting themselves to endoscopy. A procedure which allows the patient to lie supine and can be done at bedside will be most acceptable one.
The objective this study is to evaluate the role of anterior Ultrasound (USG) guided coeliac plexus ethanol neurolysis (CPN) in pain relief among patients with gastric and pancreatic malignancies.
2. Methodology
This study was carried out as an uncontrolled before-and-after non randomized trial among patients with gastric and pancreatic malignancies with abdomen pain. The study was carrie out in the Chronic Pain Management center of our tertiary care hospital, Chennai between September 2015 to March 2016. Based on the available literature, the prevalence of cancer pain was found to be 80% among patients with advanced pancreatic cancers [2]. At 95% level of significance and 15% absolute precision, the sample size was calculated as 27.3. Accounting 10% for non response, the final sample size was calculated as 30.All the patients with abdominal pain due to gastric and pancreatic malignancy with Numerical Rating Scale for pain (NRS) score of five or more; malignant pain not controlled by conventional analgesics or opioids with NRS score more than 5; and Patients who had pain relief with opioids but unable to tolerate and discontinued them were included in the study. Patient with coagulopathy or altered liver and renal function tests; patients with sepsis, local infection, patients with previous upper abdominal surgery scar which are likely to interfere with ultrasound penetration, patients with severe to moderate ascites were excluded. Approval from the Institutional Ethics Committee was obtained prior to the data collection and informed consent was obtained from the participant prior to the commencement of data collection.
The details of the patients with gastric and pancreatic cancers were obtained from the registry of the Chronic Pain Management Center. The total number of patients whose visits were scheduled between September 2015 and March 2016 were listed out. Out of 80 patients, 30 participants were selected using simple random sampling using table of random numbers.
The procedure of ultrasound guided CPN was done following a pre-assessment. During the preassessment relevant history was obtained and general examination was done. All the patients were on tramadol 200 mg orally for pain relief and seven of them were taking 20-40 mg oral morphine. Ultrasound abdomen and CT abdomen were taken to rule out invasion to vital structures. Normal coagulation profile was ascertained prior to the procedure. Analgesics were stopped on day of procedure. Participants were placed on nil per oral orders eight hours prior to the procedure. Each participant was taught about breath holding. Expected qualitative and quantitative pain relief was discussed and the anticipated complications were explained and informed consent was obtained. Numerical rating scale used for pain assessment was explained to the patient. Injection cefotaxime 1gm was administered one hour prior to the procedure as a prophylactic antibiotic.
Participants were given USG guided diagnostic trial coeliac plexus block with 10 cc of 1% preservative free lignocaine. The USG guided anterior coeliac plexus ethanol neurolysis was done as a bedside procedure after successful diagnostic block. Participant was placed in a supine position, i.v. access obtained and preloading with one litre of RL is done. ECG, NIBP, SPO2 were connected. Strict aseptic precautions for preparation of parts and sterile draping for the USG probe were followed. A SIUI ultrasound machine having a convex transducer of 3-5 Mhz is used for this procedure. Probe was placed in the epigastric region in a transverse orientation. Aorta and the vertebrae were identified. Confirmation of the aorta was done by color flow monitor and power doppler. Transducer was moved obliquely upwards and the coeliac trunk was identified as a branch of artery originating anterior from the aorta. Tilting of the probe enabled the view of the common hepatic artery and the splenic artery, and by placing the transducer in a longitudinal direction, we are able to visualize aorta and its coeliac trunk in longitudinal section. With the probe in transverse orientation, local anesthesia with 2 % lignocaine 4 cc was given and a 22G 15 cm needle is introduced through an out of plane sonographic technique. Once the needle tip was confirmed near the target area, which can be either side of the coeliac trunk, using hydro localisation with 5ml of saline, 10 ml of 1% lignocaine without preservative was injected after negative aspiration of blood and 20ml of 60% ethanol was injected. Spread of ethanol around the coeliac trunk was confirmed sonographically observed hydrolocalization [5]. Dexamethasone 8mg and 2 ml of 1% lignocaine were given before withdrawing the needle. Patient was monitored for 3 hrs before shifting to the ward. Numeric rating scale (NRS) for pain was recorded immediately after injection, 24 and 72 hrs post injection. Patient wase instructed to report after 1 week and also at 3 months for evaluation of pain.
Numeric rating scale (NRS) [6] for pain is used to assess the pain intensity. It is a segmented numeric version of visual analogue score that best reflects the intensity of pain. It is a 11 point numeric scale starting from 0 representing no pain and 10 representing the worst possible pain [6] .The block was considered successful if the NRS pain score was reduced by four points and the duration of pain relief was for more than four hours.
Data was entered and analysed using Statistical Package for Social Sciences (SPSS) ver 16.0 software. Paired sample t test was used to analyse the difference between two related variables.
3. Results
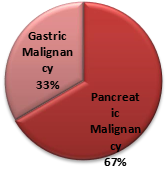
This study was done as a before-and- after study among gastric and pancreatic cancer patients. A total of 30 patients were analysed of which 25 were men and 5 women with a mean age of 44 years. Out of the 30 patients, 20 (66.6%) had pancreatic malignancy. The distribution of the study participants is given in Figure 1.
Figure 1. Distribution of the study participants.
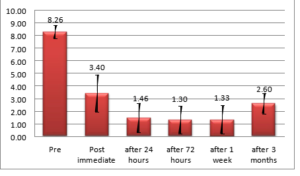
Twenty five patients had good pain relief immediately, 24 hours, 72 hours, one week and three months after the procedure. Three patients reported neurolytic pain immediately but reported good pain relief after that episode. Two patients had moderate pain and are continued morphine till the study period. There was a statistically significant difference between pre-NRS and post immediate NRS (mean 8.26 \(\pm\)0.52 and 3.40 \(\pm\)0.47)(P value \(< 0.0001\)) and significant decrease in NRS was seen after 24 hours, 72 hours, 1 week, and 3 months. The pre and post procedure mean NRS scores are given in Figure 2.
Figure 2. NRS Score From Pre-Procedure Up To Three Months post-procedure.
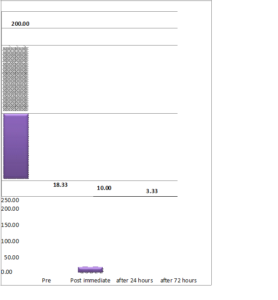
The mean dosage of tramadol consumption pre and post procedure is given in Figure 3. It was observed that the tramadol consumption drastically reduced from pre- to immediate post procedure and further reduced beyond 72 hours, the results were statistically significant p-value\((< 0.0001)\).
Figure 3. Tramadol consumption from pre-procedure upto three months post procedure.
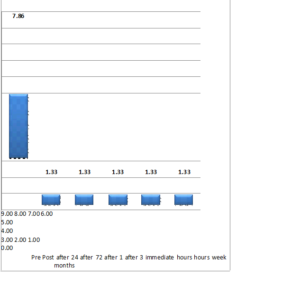
The mean dosage of oral morphine consumption pre and post procedure is given in Figure 4. It was observed that the morphine consumption drastically reduced from pre- to immediate post procedure, the results were statistically significant p-value\((< 0.007)\).
Figure 4. Oral Morphine Consumption from pre-procedure upto three months post procedure.
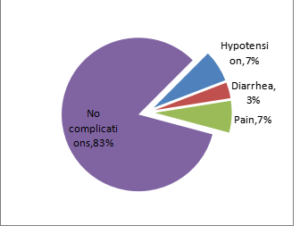
The prevalence of immediate complications of the procedure is given in Figure 5. Hypotension and procedural pain were the most common complications recorded (7%). Diarrhea was also reported (3%). However, no complications were observed in 83% of the participants.
Figure 5.Complications Documented During The Study.
4. Discussion
WHO advocates pain relief as a right to cancer patients. Patients who are suffering from cancer related pain expects relief from pain as an important and desirable aspect of cancer management. CPN with USG guidance has proven to be a better way to decrease the chronic abdominal pain associated with upper abdominal malignancy.Pancreatic and gastric malignancy patients can have pain relief by having oral analgesics or morphine. In most of the cases their oral therapy is not adequate and so patients need to undergo interventional pain management like coeliac plexus neurolysis as done in this study [1].Coeliac plexus neurolysis is mostly done under fluoroscopic method but USG guided, anterior percutaneous approach is gaining acceptance. This procedure is equally efficient in reducing pain as that of fluoroscopy guided posterior approach [5]. Being done under real time it can minimize the possibility of intravascular injections and USG guidance facilitates ease of deposit of the drug around the coeliactrunk [2]. The coeliac plexus is found more consistently around coeliactrunk rather than first lumbar vertebrae, which the fluoroscopy guided technique uses as its landmark. So its pain relief is found to be statistically significant. The other advantage of an ultrasound guided procedure is that there is adequate scope to view the organs that we traverse during the injection. Being done in supine position it minimizes the discomfort of lying prone during fluoroscopic method and this is the most important aspect as this will increase the patient cooperativeness when doing the procedure. In our study it is done as a bedside ICU procedure thus eliminating the need for having a dedicated space and it brings down the logistical time required to shift the patients for the procedure [7].
Ultrasound guided intervention eliminates radiation to the performer and the patient. This can augment the acceptance of the procedure by the patient and the physician.Adequately treated pain can improve the physical and emotional wellbeing of the patients. The ultrasound guided CPN is a safe and effective method of reducing pain of upper abdominal cancer with no major complications and high success rate [5]. Our study results found to be similar with [7]. The authors in [8] showed celiac ethanol neurolysis is more effective in reducing pain and leads to decreased opioid requirements and thus their related side effects, and thus preventing deterioration in quality of life in such patients.
The sonographic CPN is an efficient, safe, and fast method for relieving pain in the upper abdominal malignancy. The use of ultrasound helps in real time needle placement, and helps to examine the drug spread around aorta. Our study also found that USG guided real time imaging is an advantage [2].
Our study shows similarity with percutaneous neurolytic coeliac plexus block by Ashley M. Nitschle in documenting significant improvement of abdominal pain and decreased narcotic use and in reporting major complications. This study also shows similar results in terms of patient comfort, direct visualization of Vascular structures. It also documents that traversing abdominal structures including bowel and liver is generally well tolerated [9].
Kambadakone et al., [7] observed Celiac plexus neurolysis does not completely abolish pain; rather, it diminishes pain, helping to reduce opioid requirements and their related side effects and improving survival in patients with upper abdominal malignancy. We concur with this study [3]. Marcy et al., [8] have reported utility of the anterior approach and the real time colour ultrasound guidance in cancer patient lead to 93% success rate for ultrasound guidance in comparison with 100% for CT-guidance. Our study success rate is also similar. Davies [10] in their information obtained from questionnaire to the patient who underwent neurolytic coeliac plexus block over a period of 5 years found that incidence of major complication is one case per 683 blocks. Eisenberg et al., [11] have concluded in their meta analysis of neurolytic coeliac plexus block for cancer pain that NCPB have long lasting benefit in 70-90% of patients with pancreatic and intraabdominal cancers and severe adverse effects are uncommon.
5. Conclusion
USG guided anterior percutaneous Ethanol CPN is effective in reducing chronic abdominal pain of gastric and pancreatic malignancy. It will increase the patient cooperativeness by allowing them to be supine and in a .bedside setting. For the performer it is mostly a single puncture technique, giving real time view of vascular structures and abdominal organs and its complication rates are low. It gives statistically validated improvement in pain relief and subsequent physical and emotional improvement for the patient. Since radiation hazard is eliminated, we can hope that this intervention technique will be embraced more both by the patient and physician.Author Contributions:
All authors contributed equally to the writing of this paper. All authors read and approved the final manuscript.Conflicts of Interest:
''The authors declare that they do not have any competing interests.''References
- Dikshit, R. P., Mathur, G., Mhatre, S., & Yeole, B. B. (2011). Epidemiological review of gastric cancer in India. Indian Journal of Medical and Paediatric Oncology, 32(01), 3-11. [Google Scholor]
- Tadros, M. Y., & Elia, R. Z. (2015). Percutaneous ultrasound-guided celiac plexus neurolysis in advanced upper abdominal cancer pain. The Egyptian Journal of Radiology and Nuclear Medicine, 46(4), 993-998.[Google Scholor]
- Caratozzolo, M., Lirici, M. M., Consalvo, M., Marzano, F., Fumarola, E., & Angelini, L. (1997). Ultrasound-guided alcoholization of celiac plexus for pain control in oncology. Surgical Endoscopy, 11(3), 239-244. [Google Scholor]
- Sehgal, S., & Ghaleb, A. (2013). Neurolytic celiac plexus block for pancreatic cancer pain: a review of literature. Indian Journal of Pain, 27(3), 121--131. [Google Scholor]
- Ghai, A., Kumar, H., Karwasra, R. K., Kad, N., Rohilla, S., & Parsad, S. (2019). Ultrasound guided celiac plexus neurolysis by anterior approach for pain management in upper abdominal malignancy: Our experience. Anaesthesia, Pain & Intensive Care, 19, 274-281. [Google Scholor]
- Nitschke, A. M., & Ray Jr, C. E. (2013, September). Percutaneous neurolytic celiac plexus block. In Seminars in Interventional Radiology (Vol. 30, No. 03, pp. 318-321). Thieme Medical Publishers. [Google Scholor]
- Kambadakone, A., Thabet, A., Gervais, D. A., Mueller, P. R., & Arellano, R. S. (2011). CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. Radiographics, 31(6), 1599-1621. [Google Scholor]
- Marcy, P. Y., Magne, N., & Descamps, B. (2001). Coeliac plexus block: utility of the anterior approach and the real time colour ultrasound guidance in cancer patient. European Journal of Surgical Oncology, 27(8), 746-749. [Google Scholor]
- Asghari, A., Julaeiha, S., & Godarsi, M. (2008). Disability and depression in patients with chronic pain: pain or pain-related beliefs?. Archives of Iranian Medicine, 11, 263-269. [Google Scholor]
- Davies, D. D. (1993). Incidence of major complications of neurolytic coeliac plexus block. Journal of the Royal Society of Medicine, 86(5), 264-266. [Google Scholor]
- Eisenberg, E., Carr, D. B., & Chalmers, T. C. (1995). Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesthesia & Analgesia, 80(2), 290-295. [Google Scholor]