Engineering and Applied Science Letter
ISSN: 2617-9709 (Online) 2617-9695 (Print)
DOI: 10.30538/psrp-easl2022.0080
Effects of textile dyeing effluent on the environment and its treatment: A review
Pranay Dutta\(^{1,*}\), Md. Razaya Rabbi\(^{2}\), Mohammad Abu Sufian\(^3\) and Shahnaz Mahjebin\(^{4}\)
\(^{1}\) Department of Textile Technology, Chittagong Technical College, Chattogram, Bangladesh.
\(^{2}\) Department of Merchandising, Opex and Sinha Textile Group, Narayangonj, Bangladesh.
\(^{3}\) Department of Wet Processing, Textile Engineering College, Zorargonj, Chattogram, Bangladesh.
\(^{4}\) Department of Industrial Engineering, Regency Garments Ltd., Chattogram, Bangladesh.
Correspondence should be addressed to Pranay Dutta at pranaydutta992@gmail.com
Abstract
Keywords:
1. Introduction
The textile industry is indeed one of the world's most significant sectors. This sector employs people without any extraordinary skill, which plays a crucial role in employment in developing countries, namely, Bangladesh, Vietnam, Pakistan, Sri Lanka, and India. Hence, it plays a significant role in developing the value of the Gross Domestic Product (GDP) of these countries [1]. Moreover, due to the rising demand from the population, textile goods have increased, and textile mills and their wastewater have risen proportionately, leading the planet to have a big pollution problem. Since the textile industry represents two-thirds of the total demand for dyes, approximately 10-15% of the used dyes are discharged into the wastewater during the dyeing phase [2]. Therefore, T the textile industries are one of the world's leading causes of various pollution. Besides, more than 1,000,000 synthetic dyes are generated worldwide with an annual production of approximately \(7 \times 105\) metric tons [3,4], and these dyes are widely used in the textile, paper, pharmaceutical, food, and cosmetics industries [5]. However, the textile industries are the largest consumers of dyes [6]. The World Bank estimates that approximately 20% of global industrial water pollution comes from the treatment of wastewater and dyeing of textiles. Thus, the textile industries are second to agriculture practices as the biggest polluting agents for freshwater bodies.
The effluents contain heavy metals, trace metals, coloring agents, and some toxic elements. The effluent discharged into the rivers goes far away and is used by people for their day-to-day activities, and irrigation [7]. Consequently, the physicochemical parameters of water such as pH, BOD, COD, TDS, DO get degraded owing to the polluted water. It will contaminate the food chain and ecosystem. These make water very toxic and harmful for humans, crops, and aquatic livings. This causes severe diseases such as cancer, damage of infants' brains, body shrinkage on human beings, reduces soil fertility, and damages crops [8].
These days, textiles are dyed with aromatic and heterocyclic dyes. Dyes are more challenging to degrade in textile effluent because various chemical and physical materials are found in textile waste. The unfixed dyes in textile effluents were considered to be in massive amounts. These unfixed dyes with used water are mostly dumped into various nearby water sources, which are known as textile wastewater, or effluent [9]. As industrial effluents or wastewater are dumped directly into sewage systems without treatment from most factories, the sewage systems are directed into canals, which discharge their contents into rivers and lagoons. The result of this is the contamination of surface water, which has a consequent impact on human wellbeing. Industrial effluents have been reported to contaminate water, soil, and air, resulting in high disease burdens and, ultimately, a shorter life span in developed countries [10]. The environmental hazard due to wastewater is alarming for developing countries like Bangladesh. Many international investors are now aware of environmental pollution and looking for whether textile factories have ETP or not for the safe emission of effluents. Hence, the industries should be aware of the effect of polluted effluent in the environment and the threat it has on human life. Before dumping dyeing wastewater into our surroundings, these dyes must be eliminated from industrial effluents. The study was done to offer a complete overview of dye categorization, environmental impacts, their detrimental consequences, and the different techniques for removing dyes from textile dyeing effluent, followed by the findings of numerous investigations.
2. Water consumption and discharge of the textile effluent into Aquatic ecosystem
Textile industries significantly contribute to freshwater pollution, especially cotton and cotton-based fabric, the main culprits. A 2017 study estimated that 79 billion cubic meters of water were used by the apparel industry alone in 2015; this amount would be sufficient to replenish 32 million Olympic-sized swimming pools. By 2030, the number is projected to grow by 50%. As the earth's water resources are running low, this is a staggering volume [11].Furthermore, the entire demand for clothes is expected to climb by 63% from 62 million tons now to 102 million tons in 2030 if the world's population expands to 8.5 billion people as forecasted by 2030 [12]. In other words, the apparel market is growing at an unprecedented pace. It takes roughly three thousand liters of water to manufacture only one cotton shirt. About 93 billion cubic meters of water are used annually for textiles production (including cotton cultivation), accounting for 4% of the total global freshwater extraction [13].
Nearly 4,560 textile factories may be found in Bangladesh. There are currently 500-700 wet processing plants in operation, and the quantity is increasing daily [14]. In Bangladesh, it was anticipated that around 1,700 wet processing units were devoted to textile washing, dyeing, and finishing, and textile factories consume an estimated 250-300 liters of water for each kilogram of cloth produced. Which is the equivalent of two people's daily water use [15]. Some simple activities, such as sizing, use less water, while others, such as dyeing many washes and rinsing, need a large number of consecutive operations. The amount of water used depends on the type of material being treated and the final finish. The dyeing process and numerous activities and energy production, namely, steam generation, cooling water, sanitation, all, use water.
However, annually, the textile industry dumps massive amounts of dyeing effluent into our waterways. It has been calculated that a single factory may utilize two hundred tons of freshwater for each ton of colored cloth. This dye-based wastewater is dumped into Bangladesh's neighboring rivers, mostly in untreated conditions, gradually expanding into the sea. It leads to the use of highly poisonous chrome, which indicates a drastic increase in these countries' diseases [13].
2.1. Textile Dyes
A dye is a coloring substance used to add color to various substances or modify something's color. Dyes contain chromophores and auxochromes that are accountable for their substantiveness and color. Textile dyes are mostly synthetic chemical compounds with an aromatic structure, and these synthetic dyes are always responsible for decreasing light penetration and disrupting photosynthesis in the aquatic ecosystem. In other words, we can say, 'Dyes are detrimental for aquatic species.' Dyes that are employed by the textile industry presently, for the most part, are synthetic. The majority of them are extracted from two sources: coal tar and fossil fuel intermediates. Powders, granules, pastes, or solvent dispersions are sold as most of these dyes. These latest dyes are frequently formulated to satisfy newer technology's needs, new fabric styles, detergents, advancements in dyeing equipment and address the significant environmental problems raised by certain current dyes. The fact that nearly all goods are subject to seasonal demand and variations is also another significant consideration. In order to satisfy these all modern and unique technological specifications, industrial textile dyes must expand. Since the textile industry's product quality is being frequently changing, the trend of using these dyes is also changing rapidly, ranging from durable, flexible synthetic fibers to high-cost cellulosic fibers [16].2.2. Types of dyes and their brief properties
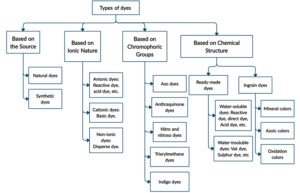
Dependent on numerous parameters, dyes can be categorized. We can, nonetheless, have a look at the four most noticeable ones in Figure 1.
Figure 1. Classification of dyes.
2.2.1. Direct Dyes
As they have a good affinity, we may add these dyes directly to the fabric, called direct dyes. This dye is predominantly a sodium salt of sulphonic acid or carboxylic acid, with azo as their leading chromophoric group. They are soluble in water and are anionic. It is common to color cellulosic fibers; protein fibers are also used. The fastness properties are average; the wet fastness is predominantly low, which means this kind of direct dye produces a wide range of wastewater during the dyeing process. Thus, the after-treatment process is required for enhanced consistency. It is generally used for dyeing viscose, and cotton-related goods [17,18].2.2.2. Reactive Dyes
By forming a covalent bond, halogen-containing reactive groups are present in reactive dyes and become part of the fiber structure. This dye is often used to color cotton fabrics. It is soluble in water and anionic in nature and shows strong wash fastness, which means less responsible for producing wastewater in volume than other dyes. In addition, this dye has a covalent bond, helping it lock into the fabric more firmly. Generally, it is used for dyeing cotton goods; it is also possible to dye protein, and polyamide-based products [17,18].2.2.3. Basic Dyes
They are primarily organic-based salts. For color production, this dye structure contains cationic charges responsible for that. That is why they are called cationic dyes occasionally. This dye is easily soluble in methylated spirit and alcohol, but not water. It is mainly used for acrylic, and jute-related goods [17,18].2.2.4. Vat Dyes
In their composition, these dyes typically consist of a keto group and are rendered water-soluble by vatting. Vat dyes are very similar to sulfur dyes in their application procedure. They are used primarily to dye denim or jeans. However, they are natural substances for coloring and are not soluble in water. For this reason, to turn them into a water-soluble form, the vatting process is required, and alkaline conditions are necessary for vatting. It is generally used for dyeing cotton-based goods [17,18].2.2.5. Disperse Dyes
Dyeing thermoplastic hydrophobic fabrics with these dyes are done, as well as these dyes are not easily water-soluble. The dispersing agent is essential for water dispersion to be produced. They have little attraction for any fiber. Additionally, these synthetic fabric dyes are primarily replaced by azo, anthraquinone, or diphenylamine compounds. It is mainly used for dyeing acetate, triacetate, nylon, acrylic, polyester-related goods [17,18].2.2.6. Acid Dyes
Acid dyes are mainly carboxylic, or sulphuric acid salts, highly soluble in water and anionic, and they form ionic bonds primarily, but van-der-Waals and H-bonds are formed as well. In addition, acid dyes are applied in an acidic bath; they can dye polyamide and protein fibers, as this dye is substantially effective on these fibers. It is primarily used for dyeing protein fibers and polyamide-based products. Dyeing thermoplastic hydrophobic fabrics with these dyes are done, and these dyes are water-soluble.2.2.7. Azoic Dyes
These were mainly mono or bi-azo water-insoluble coloring substances that need coupling components to produce colors. Unlike other dyes, the most remarkable aspect was that they were not ready-made colors. Furthermore, their structure possesses an insoluble azo group and is not water-soluble; their preparation needs two baths, such as a developing bath and an impregnation bath; diazonium and coupling compounds affect their color. As a result, there is diversity in their application to textile industries. Cotton, nylon, and polyester-related materials are commonly used with these dyes [17].2.2.8. Mordant Dyes
These dyes, also known as chrome dyes because these dyes primarily consist of inorganic chromium, possess no affinity towards textile materials. Instead, chemical binding agents, called mordants, assist them in sticking to the fiber. It is mainly used for dyeing natural protein fibers, nylon, and modacrylic fibers [17].2.2.9. Sulfur Dyes
The sulfur dye used to produce black and brown cotton fabrics is analogous to vat dyes. They contain a disulfide linkage in their structure. However, they are not soluble in water, so they need reducing agents to make them soluble. Furthermore, it must be used in an alkaline medium, and oxidation is essential for the production of color. Generally, it finds particular applications in the dyeing of silk, paper, and certain types of leather and is used in limited quantities in the dyeing of cellulosic materials [17].2.2.10. Anthraquinone Dyes
There are two significant classes of textile dyes: anthraquinone dyes and azo dyes. In terms of color range, these dyes cover nearly all visible spectrum. Moreover, as anthraquinone-based dyes possess glued aromatic structures, they resist degradation for a long time [17].3. Textile operations
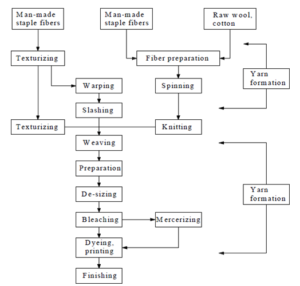
Pretreatment, dyeing/printing, finishing, and other technologies are included in textile printing and dyeing. Various methods are employed in the pretreatment stage, such as de-sizing, scouring, washing, and more. The primary goal of dyeing is to dissolve the dye in water, which will then be imparted to the cloth to make a colored cloth, given specific conditions. Printing is a type of dyeing known as "localized dyeing," which refers to dyeing that has been restricted to a specific area of the fabric. For the most part, the reactions involved in this dyeing are identical to those involved in dyeing. As opposed to printing, which uses a thick paste of colorant, dyeing employs liquid colorants. Finishing procedures are applied to both natural and synthetic textiles. As a result, various finishing agents are employed to soften, cross-link, and waterproof the completed cloth. Every step of the finishing process causes water pollution. Before dying or printing should have been done the following processes: singeing, mercerizing, base reduction, and others. Bleaching is a critical step in the textile dyeing process, and Bleaching using sodium hypochlorite or sodium chlorite is the most popular method. Also, chlorine dioxide, a strong oxidant, is corrosive and poisonous. The usual quantity of bleaching effluent is between 10 and 200 mg/L. Figure 2 depicts a typical printing and dyeing procedure.
Figure 2. A cotton mill’s several processing processes for textiles [20].
Table 1. Unfixed dye percentage for distinct forms of dye and applications [16].
| Polyester | Disperse | 8-20 |
|---|---|---|
| Wool, Viscose, and Cotton | Sulfur dyes | 30-40 |
| Reactive dyes | 20-50 | |
| Azoic dyes | 5-10 | |
| Vat dyes | 5-20 | |
| Direct dyes | 5-20 | |
| Pigment | 1 |
After the dyeing process, water used in the whole process, extracted from textile products, is named wastewater or effluents. We will find different chemical or physical substances and a large proportion of unfixed dyes from those extracted water. These dyes are the main culprits for wastewater formation because it is not relatively easy to extenuate unfixed synthetic dyes from wastewater than other chemicals. Therefore, we need to know the percentage of unfixed dyes. It varies depending on the dyes and raw goods where these dyes are generally applied. From Table 1, we will know which types of dyes are more responsible for textile wastewater formation, depending on their dye wash-out percentage after dyeing.
4. Characterization of textile wastewater
Textile mills could be divided into two categories based on waste and wastewater generation: wet processing and dry processing. The wastewater generated by the textile industry includes cleaning wastewater, process wastewater, noncontact cooling wastewater, and steam water. In dry processing units, generally solid waste is generated, and in wet processing units, most textile wastewater is generated. Raw textile dyeing effluent can be characterized by BOD, COD, TDS, color, total suspended solids (TSS), heavy metals, etc. The textile wastewater exhibits a wide range of pH (2-14), COD (50 ppm - 18000 ppm), TDS (50 ppm - 6000 ppm), and very strong color [20]. Textile wastewater is challenging to treat because of its variations in some of the factors, namely, pH, color, temperature, TDS (Total dissolved solids), Total suspended solids (TSS), and dissolved solids (DO) (see Table 2) [21].
Table 2. Characterization of textile wastewater [21] .
| Category | BOD (ppm) | COD (ppm) | pH | Temp. |
|---|---|---|---|---|
| High | 500 | 1500 | 10 | 28 |
| Average | 270 | 970 | 9 | 28 |
| Low | 100 | 460 | 10 | 31 |
4.1. Main characteristics of textile dye effluents
4.1.1. Color
Color present in the dyes is responsible for inhibiting the self-purification potential of dye effluent by reducing the photochemical synthesis of oxygen and disturbing the ecosystem. Furthermore, when dispersed in water, wastewater containing dye causes very harmful effects on aquatic life as the color present in dye effluent absorbs sunlight required mainly for aquatic plants. Which in return impacts less dissolved oxygen in wastewater, which is ultimately harmful to the aquatic animals. Hence, color is one of the critical characteristics of textile wastewater that must be treated before discharge from industries.4.1.2. Dissolved solids
Dissolved solids are another essential characteristic of dye effluent. High TDS dye effluents have a lot of disadvantages, such as high TDS leading to disturbing the surface water quality of water. Therefore, high TDS water is not suitable for use as raw water for industry and is not suitable for irrigation.4.1.3. Chlorine
The chlorine present in effluent wastewater is harmful to the water bodies. Chlorine is a toxic gas that irritates the skin, eyes, and respiratory system. The presence of chlorine in effluent reduces the dissolved oxygen content in water. Chlorine reacts with another compound to form complex chlorine salt.4.1.4. Organic materials
Organic pollutants include pesticides, fertilizers, hydrocarbons, phenols, detergents, oils, greases, pharmaceuticals, proteins, carbohydrates, etc. Toxic organic pollutants cause several environmental problems. The presence of organic pollutants is detected by analysis of BOD and COD. Most valuable oxygen is consumed to decompose the organic pollutants in textile dye wastewater.4.1.5. Toxic metals
Most of the metals are biodegradable, highly toxic, and carcinogenic. Thus removal of metals is a serious problem. Metals create adverse effects on treatment processes. Metals react with other compounds in dye in effluent and form complex metal salt, which is difficult to remove. Most of the toxic metals are chromium (Cr), cadmium (Cd), nickel (Ni), zinc (Zn), copper (Cu), lead (Pb), ferrous (Fe), etc. [4]5. Effect of wastewater on algae, fish, river water and human
The wastewater disposal exacerbates major pollution issues by some factories under unregulated and unsuitable circumstances. Undoubtedly, the urgency of prevention and treatment of pollution is crucial for human life. If a textile mill expels wastewater into the surrounding area without any treatment, it would significantly affect existing water bodies and the adjacent area's soil. In addition, strong COD and BOD5 values, the accumulation of particulate matter and sediments, and oils in the effluent allow dissolved oxygen to become reduced, which has a detrimental impact on the marine ecological environment [22].5.1. Impact textile dyes on aquatic environment
Textile dyes create a variety of environmental pollution and health risks. Due to their high thermal and photo-stability, dyes can last for a long time in the environment. The absorption and reflection of sunlight entering the water is the primary environmental problem with dyes. Besides, since cotton use has gradually grown over the last century [23], cotton fibers are mostly dyed using azo dyes, one of the major classes of synthetic dyes used in the industry [24]. Azo dye is impossible to degrade due to the ongoing conventional treatment methods. They are distinguished by the presence in the middle of the nitrogen-nitrogen bond (-N=N-) and are thus highly electron-deficient [25]. Such azo dyes are complicated and have been found to exhibit carcinogenic signs of reductive cleavage. These dyes can change the soil's physical and chemical properties, degrade the water's body, and cause disruption to the flora and fauna of the environment. It has been found that the poisonous nature of dyes leads soil microorganisms to die, which in turn influences agricultural productivity [26]. The presence of a minimal quantity of azo dye in water (< 1ppm) is prominent [27]. This affects aesthetic merit, transparency, and water-gas solubility. Reducing light penetration by water lowers photosynthetic activity, induces oxygen depletion, and deregulation of aquatic biota's biological cycles [28]. Most azo dyes are often too toxic to the environment and mutagens, which means that they can have severe chronic effects on animals, depending on the exposure period and concentration of azo dye [29].The absorption and reflection of sunlight by dyes is the main reason for light absorption decrease and the photosynthetic activity of the algae that affects the food chain. Large quantities of textile dyes in water bodies hinder the re-oxygenation of the receiving water and the sunlight, thus affecting ecological development in aquatic life and the process of photosynthesis of aquatic plants [30]. The polluting effects of dyes on the aquatic ecosystem can be toxic due to their long-term existence in the environment accumulation in sediments, particularly in fish or other aquatic species, decomposition of contaminants in carcinogenic or mutagenic compounds. Several dyes and their products are carcinogenic, mutagenic, and life-threatening. The existence of relatively tiny amounts of dyes of water is highly noticeable. It has a significant effect on the consistency and transparency of water sources, such as waterways, rivers, and others, resulting in harm to the aquatic environment [16].
5.2. Impact on human
1,4-diamine benzene is an aromatic amine whose source azo dyes can induce skin irritation, contact dermatitis, chemosis, lacrimation, exopthamlmosis, lifelong blindness, rhabdomyolysis, severe tubular necrosis, vomiting, gastritis, hypertension, vertigo. Upon ingestion, oedema of the face, throat, pharynx, tongue, and larynx along with respiratory distress [31]. Aromatic amines can be stimulated by water, which facilitates their absorption through the skin and some other exposed areas, such as the mouth. Absorption by ingestion is quicker and possibly riskier since more dye can be ingested during a shorter time frame. Water-soluble azo dyes become risky after being metabolized by liver enzymes. All those things happen when we, humans, get exposed to wastewater [29]. Due to the unavailability of fresh canal water and subsoil water, farmers have to irrigate their fields with polluted wastewater that flows through their villages. The impact on wastewater-irrigated crops can be seen through symptoms such as plaque in the villagers' teeth, knee pain, and grey hair [32].5.3. Impact on fish
Gambusia affinis, a comparative toxicological analysis of textile effluent in freshwater fish, has shown significant mortality reductions and cytotoxic effects on red blood cells (RBCs). As well as a decrease in their numbers and a percentage variance in their form (poikilocytosis) and size [33]. Another research on Mastacembelus armatus, a protensive edible freshwater fish subjected to textile effluent, has contributed to alterations in the ionic regulation of tissues such as the liver, kidneys, and muscles. The sodium and chloride ions concentration and increasing the potassium, calcium, and magnesium ions concentration [34]. The textile industry's effluent on teleost fish Poecilia recticula triggers abnormal behavior, including excessive swimming, hyper-excitation, rapid opercular activity, and thick mucus. Histopathological alters include the enlargement of the main gill bar and the detaching of the secondary gill bar. The disintegration of intestinal villi and penetration of haemocytes into the lumen has also been observed. The research was also performed on the effect of Textile-Dyeing Industry Effluent on certain Freshwater Fish Hematological Parameters Oreochromis mossambicus [35]. Hyperemia, necrosis, and degeneration are the significant histological changes found in the liver [36]. Effects of the textile dye industry effluent have also been seen on the nutritional value of the freshwater crab Spiralothelphusa hydrodrome, a crucial personal food supply in southern India, contributing to a loss of nutritional content, namely, lipids, carbohydrate, and protein [37]. Similarly, as for Catla, dye effluent strongly affects feed absorption and food conversion rates.5.4. Impact on algae
The presence of increased usage of dyes in the water bodies influences several algae parameters, such as growing protein content, pigment content, and other nutrient content. Different dyes have various possible effects on algae. When it comes to measuring pollution in aquatic environments, algae are more than half percent susceptible to pollutants than organisms commonly utilized in toxicological testing [16]. When dye concentration in water is increased, it inhibits the growth of Spirulina platensis and reduces its nutritional content. Ramazol Red Brilliant dye even affects the chain in the water bodies. It thus creates an environmental imbalance [38] The use of indigo dye is also capable of significantly lowering growth and biomass production, as well as changing the morphological structure of freshwater microalgae S. Quadricauda [39].6. An overview of the environment pollution condition of the textile industries in Banladesh perspective
Textile wet processing can be categorized as Knit dyeing units, Woven dyeing units, Denim units, Printing units [40].6.1. Knit dyeing units
Knit dyeing factory has been one of Bangladesh and South Asia's major environmentally harmful textile industries. These are most active in the manufacture of export-focused knit garments. Various forms of textile items need different treatment methods. The manufacturing technology and their treatment and testing of various fabrics are very distinct. This is because knit fabrics are softer and need to be treated gently, while other fabrics are comparatively rigid and can receive more challenging treatment options. For this cause, knitted fabrics are dyeing in a winch-type dyeing machine in which treatment occurs at a high M:L ratio of 1:150-200 means adding about 150 to 200 liters of water to dye one kg of knitted products. It was observed that a knitting factory with a production capacity of 10 tonnes consumes about 100 to 150 Million of water per hour, taking into account all the factors. All the water mentioned above, however, is not equally dangerous. Some of these are very seriously contaminated, whereas others are slightly polluted. On average, 50 percent of the water is polluted and needs to be cleaned, and the remainder of the water should be directly drained or only slightly treated. Therefore, a general guideline for knit dyeing would be that a wastewater treatment plant of 40-60 M/hrs' treatment capability can be needed for a 10-ton dyeing capacity factory [40].6.2. Printing unit
Printing-related contaminants include dissolved solids, chemicals, foam, color, and metals, and during the washing-off phases, large amounts of water are absorbed in general. Dyes containing metals, objectionable surfactants, air pollutants, water from the cleaning of the printing blanket, residual print paste, waste paste from containers, screens, and tanks. Urea use is the primary environmental contamination of textile printing as this raises the nitrogen in the effluent. Like denim, the textile printing industry's volume of effluent is minimal but heavily contaminated [40].6.3. Woven dyeing units
Woven dyed fabric gets dyed differently from knitted fabrics in a completely different way. In contrast to the knit dyeing method, the amount of wastewater produced from a woven dyeing factory is quite limited. The attributes of woven dyeing plants, in addition to this, differ from those of knit fabric dyeing plants. To maximize the strength of warp yarns, sizing is conducted before weaving. Starch is a key part of the sizing process. The wet process begins with desizing to extract the starch and other sizing substances from the cloth; otherwise, it would not be perfect for subsequent steps and dyeing. Therefore, a desizing unit heavily contaminates the emission. Starch and other sizing products, unfixed dyes, inadequate washing of dyes, machine cleaning wastes before start-up, shut-down and color and style changes, salts, alkalis, etc., are contaminants of woven dyeing units. Typically, woven dyeing is conducted at such a low M: L ratio that may be as little as 1:5 (for consistent dyeing), so the volume of wastewater is quite limited, but the degree of effluent contamination is vast [40].We already got the idea about all the different processing units in the wet process through the dyeing flowchart and the above brief description. To get a minimum idea, we will now look at probable wastewater pollutants, quantity, and nature found before and after the dyeing process (see Table 3).
Table 3. The toxic subtances found in the traditional textile dyeing and finishing plant [40].
| Processing Unit | Probable Wastewater Pollutants | Wastewater Quantity | Wastewater Nature |
|---|---|---|---|
| Sizing | Starch, waxes, carboxymethyl cellulose, polyvinyl alcohol. | Minimal volume | High BOD and COD |
| Desizing | Starch, waxes, carboxymethyl cellulose, polyvinyl alcohol, desizing, dissolved solids fats and waxes | Very small volume; | High BOD |
| (30Scouring | NaOH, Waxes, grease Na, CO3, Na2O2, and SiO2 fragments of cloth. | Small volume; Strongly alkaline; Dark color | Strongly a1kaline, |
| BOD (30Bleaching | NaOCl, Cl2, NaOH, H2O2, Acid etc. | Small volume | Alkaline constitutes |
Increasing levels of biochemical compounds and heavy metals in water due to textile industry effluent have resulted in a significant hazard to the aquatic system and public health. There are different forms of toxicity present in textile wastewater. In order to control them, the Department of Environment (DoE) has listed the most detrimental to the environment are the Biochemical Oxygen Demand (BOD), Chemical Oxygen Demand (COD), Total Suspended Solids (TSS), Total Dissolved Solids (TDS), pH, oil and grease, color and temperature. To some point, the polluting parameters of other industries can differ. The concentration of pollutants in effluents varies significantly with different textile industries. Standard wastewater parameters are given in Table 4.
Table 4. Standard wastewater parameters [41].
| Parameters on Quality of water | Unit | Standard value of discharging into the river |
|---|---|---|
| pH | --- | 6.5-9 |
| BOD | ppm | <50 |
| COD | ppm | <200 |
| TSS | ppm | <150 |
| TDS | ppm | <2100 |
| Wastewater Flow | L/Kg of fabric processing | 100 |
| Color | Co-pt unit | <200 |
| Temperature | \(^{o}C\) | <30 |
Heavy metals constituted a concern to public health and organs at the same period as well [42]. The river serves as an essential supply of water for household and commercial usage and a mode of transportation, and it helps to dilute non-biodegradable contaminants in the environment. The presence of a large concentration of pollutants, on the other hand, reduces its capacity to purify. As a result, the dilution and reoxygenation potential is significantly decreased during the dry season, resulting in a more significant presence of BOD and COD and a low presence of dissolved oxygen (DO). During floods, such contaminated water comes into touch with humans and harms the general public's health [43]. More specifically, to figure out the reason for aquatic environment pollution, we collected some data from different textile industries situated in Bangladesh that do not have any effluent treatment plant (ETP) in their factory for processing the unfixed dyed water. We endeavor to come to a firm decision by comparing our collected value with government-given standard value to understand water conditions.
Comparing Tables 5 and 6 values with the standard value of textile effluent given DoE, shows a considerable discrepancy in each parameter's values. We took only six textile factories as our research source of collecting required values and found each of them as a leading polluter and destroyer of the water ecosystem in that specific area. To carry out our research work more precisely and easily, we chose these factories situated on the same riverbank named Shitalakkha. More than 100 of them like these six factories on the same riverbank, and most of them do not have any ETP system of their own. They all prefer to release their wastewater directly into the river without minimal treatment, which occurs consistently.
Table 5. Factories that do not have an ETP plant.
| Name of the factory | BOD ppm | COD ppm | TSS ppm | TDS ppm | Color Co-pt | Temp. oC | pH |
|---|---|---|---|---|---|---|---|
| Ehsan Knitwear Ltd. | 300 | 445 | 2200 | 75 | ND* | ND | 9.3 |
| Sadmusa Knit Ltd. | 450 | 1060 | 3600 | 90 | Dark | 60 | 9.1 |
| Northwest Textile Ltd. | 325 | 1000 | 3500 | 100 | Dark | 35 | 11 |
| Purobi Knit Ltd. | 640 | 1200 | ---- | 1000 | Dark | ND | 10 |
| Brothers Denim Ltd. | 850 | 2150 | ---- | 350 | <1000 | 35 | 9 |
| Blue Denim | 640 | 1312 | 3633 | 305 | 1380 | ---- | 11 |
Table 6. Factories that have ETP plant.
| Name of the factory | BOD5 ppm | COD ppm | TSS ppm | TDS ppm | Color Co-pt | pH |
|---|---|---|---|---|---|---|
| KDS Textile Mills Ltd. | 25-45 | 60-150 | <50 | 1800-2000 | 100-150 | 7-8 |
| 4H Group | 35-48 | 70-120 | <60 | 1900-2100 | 90-120 | 6.5-8 |
| Karnafuli Polyester Dyeing Ltd. | 30-45 | 60-150 | 45-55 | 1700-2000 | 120-160 | 7-8.5 |
It can be seen that how much responsible for damaging the aquatic environment a single factory that does not have any ETP plant than a factory that have
7. Techniques for treatment of textile dyeing effluent
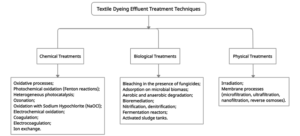
Numerous methods have been employed to find a cost-effective and reliable way to treat textile dyeing wastewater, including physical, chemical, biological, integrated treatment processes, and some other technologies (see Figure 3). Specific treatment with one of these three methods has been ineffective in extracting color and other effluents from textile wastewater. While some dyes are difficult to degrade, namely hydrolyzed reactive dyes, and some acidic dyes are not readily taken up by active sludge; thus, they are not processed. A mixture of different effluent treatment methods can eliminate over 85 percent of unnecessary matter. The resultant effluent is typically strong in color. Complementary treatment is required to eliminate color and, if possible, residual impurities. The textile industry has been condemned as the world's worst aquatic environment polluter. It requires enormous quantities of chemicals and water at any point in the textile manufacturing and finishing process. Water is required to convey the chemicals to the fabric and wash them at the beginning and end of each phase. It is packed with chemical additives and then discharged as wastewater, which pollutes the environment. Water contamination is still a significant concern in most countries. The textile industry explores an economical solution to decolor the approximately 200 billion liters of colored effluent emitted annually. Countries, governments, and companies spend billions in cash on pollution-reduction studies and the development of effluent treatment plants. The environmental concern for industrial water pollution has contributed to substantial bans on all industrial practices that pollute the environment [44]. Governments have proposed regulations restricting the volume and form of waste that may be dumped because of the adverse effects on the environment and people's wellbeing resulting from the leakage of effluent from the Textile Dye Industry [32].
Figure 3. Techniques for removal of dyes from dyeing effluent.
7.1. Chemical treatment process
An oxidative method consisting of Fenton reagent, Ozone, Photochemicals, Sodium hypochlorite, Cucurbituril, and Electrochemical destruction oxidative methods are the most widely employed chemical decoloration processes (see Table 8). This is primarily attributed to the ease of its application.
Table 7. The merits and drawbacks of chemical dye removal techniques.
| Technique | Merits | Drawbacks | References |
|---|---|---|---|
| Fenton's reagent | 1. Insoluble and soluble dyes can be effectively decolored. 2. Economical. |
1. Sludge production. 2. Excessively costly. |
[47] |
| Ozonation | 1. Gases are used. 2. Wastewater and sludge volumes are not be increased. |
1. A short half-life (20 minutes) | [48] |
| Oxidation with NaOCl | 1. Initiates and accelerates the breaking of azo bonds. | 1. Aromatic amines release. | [48] |
| Photochemical oxidation | 1. Doesn't generate sludge. 2. Low cost. |
1. By-products formation. | [49] |
| Electrochemical destruction | 1. Breakdown substances are not harmful. | 1. Electricity and operation costs are high. | [47,48] |
| Coagulation- flocculation | 1. Easy and cost effective. 2. With a short detention period and inexpensive capital expenditures. 3. Excellent removal efficiency. |
1. Sludge production is high. 2. Difficulties with handling and disposal. 3. Chemicals for pH adjustment are expensive. 4. Difficulties with dewatering and sludge handling. |
[50] |
7.2. Physicals treatments process
According to the data in Table 9, the most commonly utilized physical techniques include adsorption techniques, ion exchange, activated carbon, and membrane filtration methods (Electrodialysis, Nanofiltration, and Reverse osmosis). Adsorption techniques are quickly gaining popularity to handle aqueous effluent due to their effectiveness in eliminating too solid and commercially viable contaminants for conventional approaches. The physical process consists of activated carbon peat, wood chips, fly ash and coal combination silica gel, membrane filtration, ion exchange electro-kinetic coagulation, and other materials such as natural clay and agro-waste materials.7.3. Biological treatment process
Biological treatment is one of the environmentally sustainable and pollution-free methods using different possible organisms. The biological method consists of decoloration of white-rot fungi, various microbial cultures, adsorption of living/dead microbial biomass, and an acromial textile dye bioremediation [49]. Also, as opposed to other physical and chemical procedures, biological treatment is frequently the most cost-effective option, as illustrated in Table 9.
Table 8. The merits and drawbacks of removal techniques for dye from dyeing effluent [51] .
| \textbf{Technique} | \textbf{Merits} | \textbf{Drawbacks} |
|---|---|---|
| Adsorption | 1. High adsorption capacity for all dyes, 2. Low cost |
1. Need to dispose of adsorbents. 2. Low surface area for some adsorbents. |
| Activated carbon | 1. Removes wide varieties of dyes | 1. Very expensive, ineffective against disperse and vat dyes |
| Non-conventional adsorbents (agricultural and industrial byproducts) |
1. Effective adsorbent, inexpensive, widely available, operation is easy, process design is simple |
1. Transfer of pollutants from liquid phase to solid matrix (adsorbent) not selective |
| Membrane filtration | 1. Removes all dye types, quick method and requires less space |
1. Concentrated sludge production, membrane fouling, high cost and incapable to treat large volume |
| Ion exchange | 1. Regeneration possibility 2. The adsorbent is not lost |
1. Not effective for all types of dyes |
| Nano-filtration | 1. Separation of low molecular weight organic compounds and of divalent ions |
1. High operation costs |
| Reverse osmosis | 1. Removal of mineral salts, dyes and chemical reagents | 1. High pressure needed |
Table 9. The merits and drawbacks of biological dye removal techniques.
| Organism (procedure) | Merits | Drawbacks | References |
|---|---|---|---|
| Bacteria (aerobic) | 1. Azo and anthraquinone dyes can be decolored. 2. Biogas production. |
1. Rate of decolorization that are too low. 2. Particular oxygen catalytic enzymes are needed. 3 Additional carbon and energy sources are needed. |
[46] |
| Bacteria (anaerobic) | 1. Effective for large-scale use. 2. For sludge treatment system, it is occurred in at a pH of 7. 3. Helps both obligatory and facultative bacteria to degrade azo dyes. |
1. Toxic chemical compound production. 2. Post-treatment is needed. 3. Immobilization and recovery of redox mediator presents a challenge. |
[47] |
| Fungi | 1. Anthraquinone and indigo-based dyes decolorize at a faster pace. | 1. Azo dyes have a very low rate of decolorization. 2. A specialised bioreactor and an additional supply of carbon are required. 3. Acidic pH is needed. 4. Chemical and dye mixtures in textile wastewater inhibiting. |
[48] |
8. Techniques for removal of dyes from dyeing effluent
In an ETP, there are several ways to combine the tasks and processes:- A properly planned biological treatment plant, which usually includes screening, equalization, pH control, aeration, and settling, can effectively fulfill BOD, pH, TSS, oil, and grease demands. However, microorganisms can be harmful to the compounds in industrial effluent, so pretreatment may be essential. In addition, most dyes are complex chemicals that are impossible for microbes to degrade, so minimal color removal occurs typically.
- A Physicochemical treatment plant, usually requiring filtering, equalization, pH monitoring, chemical storage tanks, mixing unit, flocculation unit, settling unit, and dewatering of sludge, is another choice. Depending on the procedures used, this procedure will destroy much of the color. Minimizing BOD and COD will be challenging to meet effluent requirements, and it is not feasible to eliminate TDS.
- Physicochemical treatment will most commonly be associated with biological treatment. Screening, equalization, pH monitoring, storage facilities, mixing, flocculation, primary settling, aeration, and secondary settling are common components of such a plant. Physical treatment often comes before the biological units of treatment. Using a variety of treatments can usually decrease the levels of contaminants to below the standards of discharge.
- The reed bed that can be used with a settling tank or other treatment processes is another type of biological treatment. It offers a natural effluent treatment technique that is also lower in capital, service, and maintenance. Reed beds can lead to a decrease in color, a decrease in COD, an increase in dissolved oxygen, and a reduction in heavy metals, but they work better with some pretreatment [41].
Latest works on textile dye removal techniques are presented in Table 10.
Table 10. Latest works on textile dye removal techniques.
| Types of dye | Adsorbent used | pH & Temp. | Isotherms followed | References |
|---|---|---|---|---|
| Methyl Red | Adsorption by Guargum Powder | pH 4.2 & \(34^{o}\)C | Langmuir model | [52] |
| Amido black-10 B | Nano photo catalyst | --- | -------- | [53] |
| Synthetic dye | Adsorption by sago waste | pH 4 & \(34^{o}\)C | Langmuir model | [54] |
| Acid blue 92, Direct red 23, & Direct red 81 | Polymeric Adsorbent (poly amino primary secondary amine) | pH 12 | Langmuir isotherm | [55] |
| Reactive red 120 | Nano filtration poly etherimide membrane | [56] | ||
| Acid black 210 & acid red 357 | Activated carbon prepared from leather shaving wastes | pH 2 | Langmuir and BET models | [57] |
| Residual Reactive blue 49 | A coagulant and a flocculant | pH 7 \& \(60^{o}\)C | ----------- | [58] |
| Reactive red | Belpatra Bark charcoal adsorbent | \(50^{o}\)C \& pH 3 | Langmuir, Freundlich and Temkin adsorption | [59] |
9. Factors influencing the elimination of dyes
- The adsorbent's physical and chemical properties, namely, composition of chemical, area of surface, and size of pore.
- The adsorbate's physical and chemical properties, such as molecular polarity and chemical content.
- The adsorbate concentration in the liquid phase (solution).
- The liquid phase's properties, such as pH and temperature.
- The system's residence time.
- Incubation time, temperature, pH, agitation rate, and carbon, nitrogen, inorganic salts, and phosphorus sources in growth medium are all critical factors in dye decolorization in biological treatment [60].
10. Conclusion
One of the world's significant sectors is the garment and textile dyeing industry. The economy creates jobs and plays a significant role in many countries, such as Bangladesh, India, Pakistan, and Sri Lanka. Widely used in the textile industry, many synthetic dyes such as azo dye, vat dye, reactive dye, disperse dye, etc. The textile dyeing industries generate large quantities of effluent and solid waste ingredients daily. A complex combination of toxic substances, organic and inorganic, releases all these factories into the water bodies. Most pollutants are dissolved solids (DS), suspended solids (SS), colored compounds, higher BOD5, COD, and lightly alkaline, heavy metals. Most of the effluent parameters surpass the limits defined by DoE. The higher COD, TDS, TSS values indicate stronger effluent toxicity. The aquatic environment's physical, chemical, and biological natures differ due to the non-stop modification of alkalinity, odor, noise, temperature, pH, etc. Textile dyeing waste retains concentrations of metals capable of harming water, soil, and human health. Due to untreated effluents' discharge, the study found that the rivers' ecological balance in South Asia has decreased. In addition, the textile dyeing effluent seriously affects crop production in the surrounding agricultural fields. The study indicated an ETP in a few sectors, but it is not yet enough. The study revealed that some traditional treatment techniques, including flocculation, coagulation, adsorption, ozonation, and advanced oxidation procedure, are used to operate ETPs. However, a single treatment process cannot eliminate both toxic organic and inorganic pollutants from the effluent, and a series of treatment units must therefore be established.Author Contributions:
All authors contributed equally to the writing of this paper. All authors read and approved the final manuscript.Conflicts of Interest:
''The authors declare no conflict of interest.''References
- Keane, J., & te Velde, D. W. (2017). The role of textile and clothing industries in growth and development strategies (PDF). Investment and Growth Programme Overseas Development Institute (ODI). [Google Scholor]
- Khan, S., & Malik, A. (2014). Environmental and health effects of textile industry wastewater. In Environmental Deterioration and Human Health (pp. 55-71). Springer, Dordrecht. [Google Scholor]
- Ogugbue, C. J., & Sawidis, T. (2011). Bioremediation and detoxification of synthetic wastewater containing triarylmethane dyes by Aeromonas hydrophila isolated from industrial effluent. Biotechnology Research International, 2011, Article ID 967925, https://doi.org/10.4061/2011/967925. [Google Scholor]
- Chen, K. C., Wu, J. Y., Liou, D. J., & Hwang, S. C. J. (2003). Decolorization of the textile dyes by newly isolated bacterial strains. Journal of Biotechnology, 101(1), 57-68. [Google Scholor]
- Chandra, R., & Bharagava, R. N. (2013). Bacterial degradation of synthetic and kraft lignin by axenic and mixed culture and their metabolic products. Journal of Environmental Biology, 34(6), 991-999. [Google Scholor]
- Elisangela, F., Andrea, Z., Fabio, D. G., de Menezes Cristiano, R., Regina, D. L., & Artur, C. P. (2009). Biodegradation of textile azo dyes by a facultative Staphylococcus arlettae strain VN-11 using a sequential microaerophilic/aerobic process. International Biodeterioration & Biodegradation, 63(3), 280-288. [Google Scholor]
- Sarker, M. R. H., Razzaque, A., Hoque, M. M., Roy, S., & Hossain, M. K. (2015). Investigation of effluent quality from an effluent treatment plant of a textile industry, Fakir Knitwear Ltd. Narayangonj, Bangladesh. Journal of Environmental Science and Natural Resources, 8(2), 25-31.[Google Scholor]
- Munnaf, A., Islam, M. S., Tusher, T. R., Kabir, M. H., & Molla, M. A. H. (2014). Investigation of water quality parameters discharged from textile dyeing industries. Journal of Environmental Science and Natural Resources, 7(1), 257-263. [Google Scholor]
- Islam, M. R., & Mostafa, M. G. (2018). Textile dyeing effluents and environment concerns-a review. Journal of Environmental Science and Natural Resources, 11(1-2), 131-144. [Google Scholor]
- World Health Organization. (2003). The world health report 2003: shaping the future. World Health Organization. [Google Scholor]
- Benson, S. (2018, March 22). Retrieved from Refinery29: https://www.refinery29.com/en-us/water-consumption-fashion-industry. [Google Scholor]
- Thomas, D. (2019, August 29). Retrieved from The Wall Street Journal: https://www.wsj.com/articles/the-high-price-of-fast-fashion-11567096637. [Google Scholor]
- (2019, May 15). Retrieved from The Conscious Challenge: https://www.theconsciouschallenge.org/ecologicalfootprintbibleoverview/water-clothing. [Google Scholor]
- Sagris, T., & Abbot, J. (2015). An Analysis of Industrial Water use in Bangladesh with a Focus on the Textile and Leather Industries. Dhaka: 2030 Water Resources Group. [Google Scholor]
- Hasan, R., & Leonas, K. K. (2018). Collaborative approach for water & energy conservation: clothing industry of Bangladesh. Journal of Textile and Apparel, Technology and Management, 10(4), 1-11. [Google Scholor]
- Gita, S., Hussan, A., & Choudhury, T. G. (2017). Impact of textile dyes waste on aquatic environments and its treatment. Environment and Ecology, 35(3C), 2349-2353. [Google Scholor]
- El Harfi, S., & El Harfi, A. (2017). Classifications, properties and applications of textile dyes: A review. Applied Journal of Environmental Engineering Science, 3(3), 311-320. [Google Scholor]
- Muntasir, Kazi Sifat. (2020, May 15). Wet Processing. Retrieved December 28, 2020, from Textile Tuts: https://textiletuts.com/types-of-dyes/.[Google Scholor]
- Verma, A. K., Dash, R. R., & Bhunia, P. (2012). A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. Journal of Environmental Management, 93(1), 154-168. [Google Scholor]
- Babu, B. R., Parande, A. K., Raghu, S., & Kumar, T. P. (2007). Textile Technology- Cotton Textile Processing: Waste Generation and Effluent Treatment. The Journal of Cotton Science, 11, 141-153. [Google Scholor]
- Gosavi, V. D., & Sharma, S. (2014). A general review on various treatment methods for textile wastewater. Journal of Environmental Science, Computer Science and Engineering & Technology, 3(1), 29-39. [Google Scholor]
- Egli, J. (2007). Wastewater treatment in the textile industry. Pakistan Textile Journal, 56, 60-66. [Google Scholor]
- UNCTAD. (2003). Major uses of cotton fibers. Geneva: United Nations Conference on Trade and Development. [Google Scholor]
- Mohan, S. V., Rao, N. C., & Karthikeyan, J. (2002). Adsorptive removal of direct azo dye from aqueous phase onto coal based sorbents: a kinetic and mechanistic study. Journal of Hazardous Materials, 90(2), 189-204. [Google Scholor]
- Robert, L., Joseph, F., & Alexander, A. (2008). Fisher's contact dermatitis in textiles and shoes. BC Decker Inc, Ontario, 339-401. [Google Scholor]
- Savin, I., & Butnaru, R. (2008). Wastewater characteristics in textile finishing mills. Environmental Engineering and Management Journal, 7(6), 859-864. [Google Scholor]
- Chung, K. T. (1983). The significance of azo-reduction in the mutagenesis and carcinogenesis of azo dyes. Mutation Research/Reviews in Genetic Toxicology, 114(3), 269-281. [Google Scholor]
- Apostol, L. C., Pereira, L., Pereira, R., Gavrilescu, M., & Alves, M. M. (2012). Biological decolorization of xanthene dyes by anaerobic granular biomass. Biodegradation, 23(5), 725-737. [Google Scholor]
- Hassaan, M., & Nemr, A. (2017). Health and environmental impacts of dyes: mini review. American Journal of Environmental Science and Engineering, 1(3), 64-67. [Google Scholor]
- Zaharia, C., Suteu, D., Muresan, A., Muresan, R., & Popescu, A. (2009). Textile wastewater treatment by homogenous oxidation with hydrogen peroxide. Environmental Engineering and Management Journal, 8(6), 1359-1369. [Google Scholor]
- LGC. (1999). The risk of cancer caused by textiles and leather goods coloured with azo dyes. Brussels: CSTEE Plenary Meeting. [Google Scholor]
- Kant, R. (2012). Textile dyeing industry an environmental hazard. Natural Science, 4(1), 22-26. [Google Scholor]
- Soni, P., Sharma, S., Sharma, S., Kumar, S., & Sharma, K. P. (2006). A comparative study on the toxic effects of textile dye wastewaters(untreated and treated) on mortality and RBC of a freshwater fish Gambusia affinis(Baird and Gerard). Journal of Environmental Biology, 27(4), 623-628. [Google Scholor]
- Karthikeyan, S., Jambulingam, M., Sivakumar, P., Shekhar, A. P., & Krithika, J. (2006). Impact of textile effluents on fresh water fish Mastacembelus armatus (Cuv. & Val). E-journal of Chemistry, 3(4), 303-306. [Google Scholor]
- Amte, G. K., & Mhaskar, T. V. (2013). Impact of textile-dyeing industry effluent on some haematological parameters of freshwater fish Oreochromis mossambicus. Nature Environment and Pollution Technology, 12(1), 93-98. [Google Scholor]
- Sripriya, L., Vijayalakshmi, M., Sumathy, R., & Sharmila, J. (2014). The impact of textile dyes on the biochemistry and histology of liver, a freshwater fish, tilapia, Oreochromis mossambicus (Peters.). International Journal of Pharma and Bio Sciences, 5(3), 271-298. [Google Scholor]
- Sekar, P., Prasad, S. H., & Raman, M. D. (2009). Effect of textile dye industry effluent on the nutritive value of fresh water female crab Spiralothelphusa hydrodroma (Herbst). Journal of Applied Sciences Research, 5(11), 2041-2048. [Google Scholor]
- de Sousa, M. L., de Moraes, P. B., Lopes, P. R. M., Montagnolli, R. N., de Angelis, D. D. F., & Bidoia, E. D. (2012). Contamination by remazol red brilliant dye and its impact in aquatic photosynthetic microbiota. Environmental Management and Sustainable Development, 1(2), 129-138. [Google Scholor]
- Chia, M. A., & Musa, R. I. (2014). Effect of indigo dye effluent on the growth, biomass production and phenotypic plasticity of Scenedesmus quadricauda (Chlorococcales). Anais da Academia Brasileira de Ciencias, 86(1), 419-428. [Google Scholor]
- Haque, M. (2008). Treatment of textile wastewater in Bangladesh. Cotton Bangladesh, 18-24. Retrieve at: https://www.researchgate.net/publication/332440677. [Google Scholor]
- Department of Environment. (2008). Guide for assessment of effluent treatment plants in EMP/EIA reports for textile industries. Department of Environment, Ministry of Environment and Forest, Bangladesh. [Google Scholor]
- Rahman, S., & Hossain, F. (2008). Spatial assessment of water quality in peripheral rivers of Dhaka City for optimal relocation of water intake point. Water Resources Management, 22(3), 377-391.[Google Scholor]
- Bashar, T., & Fung, I. W. (2020). Water pollution in a densely populated megapolis, Dhaka. Water, 12(8), Article No. 2124. https://doi.org/10.3390/w12082124 . [Google Scholor]
- Mansour, H. B., Houas, I., Montassar, F., Ghedira, K., Barillier, D., Mosrati, R., & Chekir-Ghedira, L. (2012). Alteration of in vitro and acute in vivo toxicity of textile dyeing wastewater after chemical and biological remediation. Environmental Science and Pollution Research, 19(7), 2634-2643. [Google Scholor]
- Brahmbhatt, N. H., & Jasrai, R. T. (2016). The role of algae in bioremediation of textile effluent. International Journal of Engineering Research and General Science, 4(1), 443-453. [Google Scholor]
- Aksu, Z. (2005). Application of biosorption for the removal of organic pollutants: a review. Process biochemistry, 40(3-4), 997-1026. [Google Scholor]
- Dos Santos, A. B. (2005). Reductive Decouloristation of Dyes by Thermophilic Anaerobic Granular Sludge. Wageningen University and Research. [Google Scholor]
- Mutambanengwe, C. C. Z. (2006). Hydrogenases from sulphate reducing bacteria and their role in the bioremediation of textile effluent (Doctoral dissertation, Rhodes University). [Google Scholor]
- Zee, F. (2002). Anaerobic azo dye reduction. Netherlands: Wegeningen University. [Google Scholor]
- Sharma, S., Saxena, R., & Gaur, G. (2014). Study of removal techniques for azo dyes by biosorption: a review. Journal of Applied Chemistry, 7(10), 06-21. [Google Scholor]
- Visa, M., Cazan, C., & Andronic, L. (2014). Fly Ash Based Substrates for Advanced Wastewater Treatment. Sustainable Energy in the Built Environment-Steps Towards nZEB, 539-569. [Google Scholor]
- Saxena, R. & Sharma, S. (2016). Adsorption and kinetic studies on the removal of Methyl Red from aqueous solutions using low-cost Adsorbent: Guargum Powder. International Journal of Scientific & Engineering Research, 7(3), 675-683. [Google Scholor]
- Kirupavasam, E. K., & AllenGnana Raj, G. (2012). Photocatalytic degradation of amido black-10B using nano photocatalyst. Journal of Chemical and Pharmaceutical Research, 4(6), 2979-2987. [Google Scholor]
- Karthika, M., & Vasuki, M. (2018). Adsorptive Removal of Synthetic Dye Effluent Using Sago Waste as Low Cost Adsorbent. International Journal of Waste Resources, 8(2), 1-7. [Google Scholor]
- Mahmoodi, N. M., Masrouri, O., & Najafi, F. (2014). Dye removal using polymeric adsorbent from wastewater containing mixture of two dyes. Fibers and Polymers, 15(8), 1656-1668. [Google Scholor]
- Karisma, D., Febrianto, G., & Mangindaan, D. (2017, December). Removal of dyes from textile wastewater by using nanofiltration polyetherimide membrane. In IOP Conference Series: Earth and Environmental Science, 109, 1-7. [Google Scholor]
- Manera, C., Tonello, A. P., Perondi, D., & Godinho, M. (2019). Adsorption of leather dyes on activated carbon from leather shaving wastes: kinetics, equilibrium and thermodynamics studies. Environmental Technology, 40(21), 2756-2768. [Google Scholor]
- Zafar, M. S., Ahmad, S. W., Zia-Ul-Haq, M., Mubeen, A., & Khan, W. A. (2018). Removal of residual carcinogenic dyes from industrial wastewater using flocculation technique. Chemical Industry and Chemical Engineering Quarterly, 24(1), 69-76. [Google Scholor]
- Gupta, V., Agarwal, A., Singh, M. K., & Singh, N. B. (2017). Removal of Red RB dye from aqueous solution by belpatra bark charcoal (BBC) adsorbent. Journal of Materials and Environmental Sciences, 810, 3654-3665.[Google Scholor]
- Eid, A., & Prol, A. (2017). Adsorption and Bioremediation as Technologies of Wastewater Treatment. (Doctoral dissertation, School of Chemical, University of Almonifia). [Google Scholor]