Nicotiana tabacum is rich with medicinal importance. The aim of the study is to isolate, purify and compare nicotine (3-[1-Methylpyrolidine-2 yl] pyridine) from different brands of cigarettes. Isolation is carried out by liquid-liquid extraction using ether as solvent. Crude nicotine samples are further purified with SPE (solid phase extraction) method. Nicotine crystals are complexed with zinc chloride (\(ZnCl_{2}\)). Fine and pure crystals of metal complexes are obtained with Nic-C and Nic-D. However, no complex formation is synthesized with Nic A and Nic-B due to impurities in the sample which is confirmed by thin layer chromatography. Antibacterial activity of the samples is carried out against different strains of bacteria which showed positive results for Nic-C and Nic-D. The outcomes of the study reveal that Zinc-Nicotine complexes can be used as therapeutic agents and anti-sickness agents in sickle cell disease.
Nicotiana tabaccum, is a stout herbaceous plant in the solanaceae family that originated in the tropical Americas and now cultivated worldwide [1]. It is the commercial source of tobacco. It contain nicotine, a powerful neurotoxin that is particularly harmful to insects [2]. Nicotine, a carcinogen, is the compound responsible for the addictive nature of tobacco use [3]. Nicotine is also used as insecticides. Structure of nicotine is shown in Figure 1.
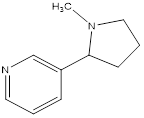
It was observed by literature that nicotine could be extracted by different techniques. Nicotine and zinc are closely related to a variety of brain pathologies, including schizophrenia, anxiety, depression, Parkinson’s and Alzheimer’s diseases [4]. Yuegang Zuo developed ultrasonic extraction (UE) of nicotine with heptane followed by direct capillary gas chromatography (GC) separation [5]. Lubomir Svorc developed a sensitive, selective and reliable electrochemical method for the determination of nicotine using differential pulse voltammeter on a bare boron-doped diamond electrode [6]. We have used the technique which is not used in previous studies. Our method is more economical and convenient. Nicotine was isolated from the seeds of N. tabacum and its Zinc (II) complex was synthesized [7] and now in the present study, nicotine is isolated from different brands of cigarettes and its Zinc complexes are synthesized and bacterial sensitivity against different micro-organisms were performed. The outcomes of the study reveal that Zinc-Nicotine complexes can be used as therapeutic agents and anti-sickness agents in sickle cell disease.
| Sr.No. | BrandName | Abbreviation | Company | Price / Cig | Total Price/20cig |
|---|---|---|---|---|---|
| 1 | Prime | Nic-A | Flacon CigaretteIndustry | 2 | 42 |
| 2 | Cafe | Nic-B | Universal TobaccoCompany | 2.5 | 50 |
| 3 | Classic | Nic-C | National TobaccoIndustries | 3 | 58 |
| 4 | Dunhill | Nic-D | Dunhill Tobacco ofLondon Limited | 7.5 | 150 |
Digital melting point apparatus is used to determine melting points of different extracted pure samples (after SPE Purification). The average melting point of the samples was \(-79C^0.\) Thin layer chromatography is subjected to check the specificity and purity of nicotine in all brands by using solvent system n-butanol: acetic acid: water (3:1:1 v/v) and compared it by Co-TLC with the standard. It was found that the nicotine in all the brands showed single spot (\(R_f\) values 0.47) after visualization on TLC plate which confirmed the purity and specificity of the nicotine and it is also noted that no interference of any component on TLC observed (Table 2). FT-IR spectroscopy was used for IR-spectrum of extracted purified nicotine i.e. shown in Table 3. By using an UV-absorption spectrophotometer, absorption spectrum of extracted purified crystalline nicotine is taken at different absorbance against different wavelength. The \(\lambda_{\max}\) was found to be 760nm.
| Sample | Melting Point(\(C^{0}\)) | \(\lambda_{max(mn)}\) | Rf |
|---|---|---|---|
| Nic-A | -80 | 759 | 0.46 |
| Nic-B | -78 | 758 | 0.48 |
| Nic-C | -79 | 760 | 0.47 |
| Nic-D | -79 | 760 | 0.47 |
| Bond | v(\(cm^{-1}\))Nic-A | v(\(cm^{-1}\))Nic-B | v (\(cm^{-1}\))Nic-C | v(\(cm^{-1}\))Nic-D |
|---|---|---|---|---|
| CH (Stretching) | 2964 | 2963 | 2968 | 2970 |
| C=N | 1673 | 1674 | 1676 | 1677 |
| C=C | 1689 | 1689 | 1690 | 1691 |
| CH (Bending) | 902 | 901 | 903 | 904 |
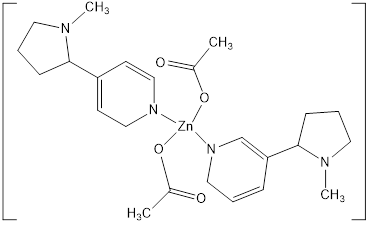
In our present study, four different brands of cigarettes i.e., Nic-A, Nic-B, Nic-C and Nic-D of four different companies W, X, Y and Z are used. The basic compound (Nicotine) in all the cigarettes was same with slight variation in complexation. Our first attempt was to extract and purify the nicotine alkaloid by using simple solvent extraction method and compare them to each other and with standard for qualitative analysis. Using the experimental observations, it is found that Nic-A is extracted by weight as 1.75gm in amorphous powder; Nic-B is extracted by weight as 1.46gm, Nic-C 1.07gm and Nic-D 0.87gm as fine crystals respectively.
Further the compounds are used for metal complexes, four of which, two compounds Nic-A and Nic-B could not be subjected for complex formation because of impurities were present in them, (checked by TLC with the standard). The compounds Nic-C and Nic-D are used for Zn-nicotine complex due to its purity and fine crystals. From the results and plots it is also observed and concluded that the products of company Y and Z have been better qualified than the products of company W and X. In nicotine structure due to the presence of nitrogen atom it has unsymmetrical divergent ligand property.
Here only the antimicrobial properties are discussed with reference to the standard. The antimicrobial activity of Zn-II-nicotine complex is tested against Gram negative and Gram-positive bacteria. The concentrations of the compounds are used that is 100\(\mu\)g /100\(\mu\)L and 200\(\mu\)g /100\(\mu\)L for first dose level and second dose level. Results of Zn (II)-nicotine complex for both the concentrations are given in table no. V.
| Sample | Amount of sample(g) | Amount ofNicotineafter solventextraction(mg) | %ageof Nicotinebefore SPE | %ageof Nicotineafter SPE |
|---|---|---|---|---|
| Nic-A | 10 | 1.75 | 0.0175 | 0.0125 |
| Nic-B | 10 | 1.46 | 0.0146 | 0.0100 |
| Nic-C | 10 | 1.07 | 0.0107 | 0.0098 |
| Nic-D | 10 | 0.87 | 0.0087 | 0.0077 |
| Sr.No. | Compound used | Conc. µg / 100 µL |
Gram positive organisms | Gram negative organisms |
|---|---|---|---|---|
| 1 | Gentamycin | 100200 | 3140 | 3580 |
| 2 | Tetracycline | 100200 | 1225 | 0.0125 |
| 3 | Tobramycin | 100200 | 3070 | 3050 |
| 4 | Nicotine | 100200 | 14 | 0.0098 |
| 5 | Zn-II-nicotinecomplex | 100200 | 2027 | 1820 |
The results reveal that nicotine is inactive for 1st dose level and is more effective antibiotic against 2nd dose level for Gram positive and Gram-negative bacteria i.e. S. faecalis and E. coli respectively. Zn-nicotine complex did not inhibit the both Gram negative and Gram-positive bacteria however, in the 2nd dose level; it inhibited the growth of both the bacterial species. The results are also compared with three antibiotics that is Gentamycin, Tetracycline, Tobramycin. On the basis of above evidences, we comment that this Zn-nicotine complex is broad applicant antimicrobial agent and is active against Gram positive and Gram-negative bacterial species. Further, Zinc-Nicotine complexes can be used as therapeutic agents and anti-sickness agents in sickle cell disease.