The objective of the present study was to investigate and compare the efficacy, physiochemical equivalence and purity of API’s of different brands of cetirizine 2HCl tablets (10mg) with that of multinational brands available in local market of Lahore, Pakistan. The present work also provides awareness to allergy patients about most effective local brands with reasonable prices, without any health risk. Spectrophotometric method was used for chemical assay, TLC technique was subjected for the purity of API’s and physiochemical parameters was employed i.e; weight variation, hardness, friability, thickness, disintegration time and dissolution study as well as price fluctuation in PK rupees. The results of all physiochemical parameters and chemical assay were found to be within limit and meet to the pharmacopeial standards. TLC technique was developed to check the quality and purity of API, all the active ingredients showed the similar \(R_{f}\)-values without any impurity (0.38-0.39). From the test results and plots, it was observed and concluded that an economical and quality products can be prescribed for allergy patients whether they are manufactured by the local or multinational pharmaceutical companies without any health risk.
Tablets and capsules represent unit dosage forms whereas liquid oral dosage forms such as syrups, suspensions, emulsions, solutions and elixirs usually contain one dose of medication in 5 to 30mL. Such doses are erratic by a factor ranging from 20 to 50% when the drug is self-administered by the patient [1]. The oral route of drug administration is the most important method for systemic effects. Solid oral dosage forms (tablet and capsule) are the preferred class of products of the two forms, the tablet has a number of advantages such as the tablet is an essentially tamper proof dosage form [2], [3]. Cetirizine is the carboxylated metabolite of hydroxyzine, and has high specific affinity for histamine H-receptors. Cetirizine dihydrochloride (CZ) is (RS)-2-[2-[4-[(4-chlorophenyl) phenyl methyl] piperazine-1-yl]ethoxy] acetic acid dihydrochloride. It is used for the symptomatic relief of allergic conditions including rhinitis and chronic urticarial [4], [5].
Literature survey shows comparative study of different brands of the same generic of pharmaceutical dosage form, and have indicated that pharmaceutical evaluation of different brands are as important as biological and clinical equivalency.
It is generally evaluated that many drugs that are manufactured in developing countries are implicated to be substandard [6]. To improve the quality of health products and to minimize the health risk factors. It is necessary to monitor all the pharmaceutical services in a regular basis that promoting the conditions and providing information on the base of which the people become enable to make healthy choices and they can make correct decisions about their health. Many tests are frequently applied to tablet dosage forms to render their optimum therapeutic effects. The technique of optimization is well reported in the literature for the development of tablet formulations [7, 8, 9]. The purpose of carrying out optimization is to select the best possible formulation from a pharmaceutical as well as consumer point of view.
In the context above we have made an attempt to compare all the physical and chemical parameters including purity of API’s of five different brands of cetirizine 2HCl tablet available in local market of Lahore, Pakistan with that of multinational brand on the basis of GMP, GLP and Pharmcopial standards. Since no work has so far been carried out in the said perspective. Therefore we choosed this topic and to decide to provide awareness to allergy patients about most effective local brands with reasonable prices, without any health risk.
| Sr No |
Product Name |
Menufactured by |
Batch No |
Mfg date |
Exp date |
Price/10 tab |
|---|---|---|---|---|---|---|
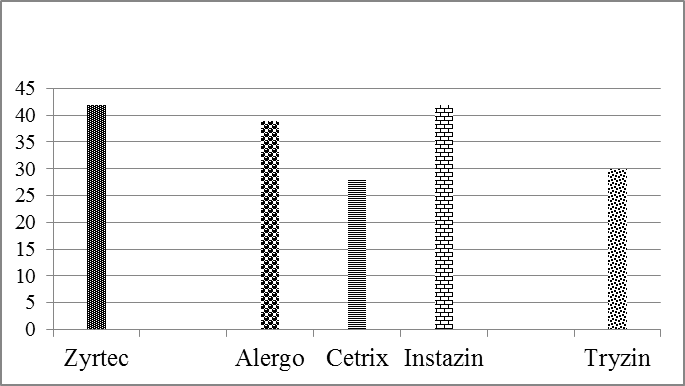
| 1 |
Zyrtec |
UCB Farchim, S.A, Switzerland | HZXAP | 04-2018 |
04-2020 |
42.00 |
| 2 |
Alergo |
Pharmix laboratories (Pvt) Ltd |
C824 | 03-2018 |
02-2021 |
39.00 |
| 3 |
Cetrix |
Saffron Pharmaceuticals (Pvt)Ltd |
106 |
11-16 |
10-19 | 28.00 |
| 4 |
Instazin | Friends Pharma (Pvt)Ltd | 18IN021 |
08-2018 |
08-2021 | 42.00 |
| 5 | Tryzin | Pulse pharmaceuticals (pvt)Ltd |
1298 |
11-2018 |
10-2021 |
30.00 |
An accurately weighed 10mg cetirizine 2HCl (reference standards) is transferred to 100mL volumetric flask and dissolved in 0.1N HCl and make up the volume up to the mark with the same solvent to obtain standard solution having concentration 1000ppm. Magnetic stirrer is used for better dissolution. Further 10ppm dissolution is made by taking 1mL from the above solution and make up the volume to 100mL with 0.1N HCl.
The standard solution of cetirizine 2HCl is made by dissolving 10mg of pure cetirizine 2HCl in 100mL of 0.1N HCl. Magnetic stirrer is used for better dissolution. Further 10ppm dissolution is made by taking 1mL from the above solution and make up the volume to 100mL with 0.1N HCl.
| Brand names |
Thickness (mm) |
Diameter (mm) |
Hardness test (kg/cm2) |
Weight variation (mg) |
Friability test (%age) |
|---|---|---|---|---|---|
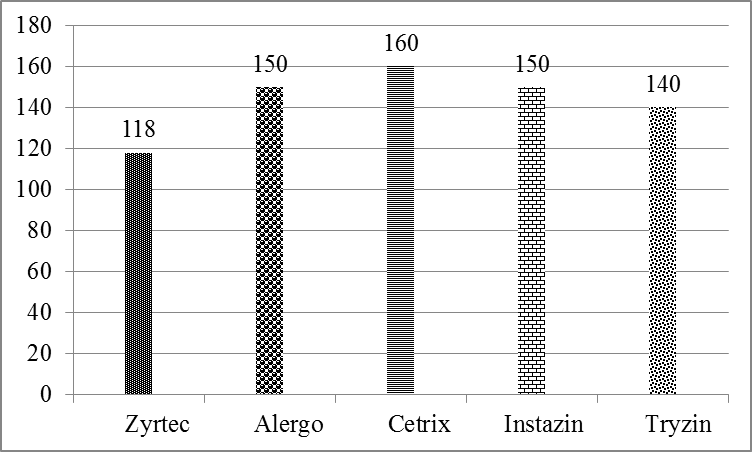
| Zyrtec |
2.71mm |
4.10mm | 3.08 kg/cm2 | 118 mg |
NMT 1% |
| Alergo |
3.51mm |
5.55mm |
3.46 kg/cm2 | 150 mg | NMT 1% |
| Cetrix |
3.08mm | 7.99mm | 2.39 kg/cm2 |
160 mg |
NMT 1% |
| Instazin | 2.90mm | 5.89mm |
3.27 kg/cm2 | 150 mg |
NMT 1% |
| Tryzin |
3.11mm | 4.03mm |
3.13 kg/cm2 | 140 mg |
NMT 1% |
| Brand names |
Disintegration time (minutes) |
\(R_{f}\) values |
Dissolution test (%age) |
Chemical assay (%age) |
|---|---|---|---|---|
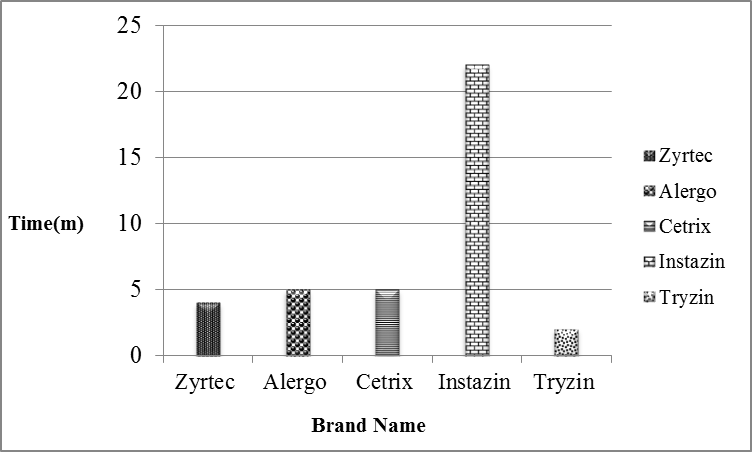
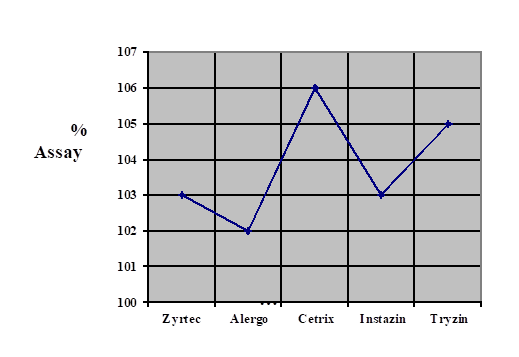
| Zyrtec |
4 minutes |
0.38 |
89.65% |
103% |
| Alergo |
5 minutes |
0.38 | 85.74 % |
102% |
| Cetrix | 5 minutes |
0.38 |
87.95% | 106% |
| Instazin |
22 minutes | 0.38 | 84.25% |
103% |
| Tryzin | 2 minutes | 0.38 | 82.70% | 105% |
Price fluctuation among different brands of cetirizine 2HCl is given in Figure 1. Weight variation comparison of different brands of cetirizine 2HCl is given in Figure 2. Comparison of disintegration time of all the five samples is shown in Figure 3 and comparison of % Assay of all the five samples is shown in Figure 4. It is evaluated by literatures that many drugs which are manufactured in developing countries are implicated to be substandard. In order to eliminate the health risk and provide the local community with affordable quality drugs, it is necessary to check the pharmaceutical services at regular basis. By providing this sort of information, people can make healthy choices according to their health problems. Relative to the quality of drug, the price fluctuation is also noticed in the societies where there is no regulatory control. In Figure. 1 the graphical representation shows that a significant variation in the cost of different brands containing same salt i.e. cetirizine 2HCl is present, whereas there is not a significant variation present in their quality. So its use can reduce the health expenses of a patient.
A drug becomes publically famous, by its effectiveness, immediate results and lesser side effects. The effectiveness in turn depends upon the content of active ingredients, disintegration time of drug, and its dissolution. All these parameters are controlled and monitored by Pharmaceutical quality control and quality assurance department. These departments check the composition and uniformity of the drug substances used in processing and in the final product. In case of above mentioned drugs from local pharmaceuticals, the traditional tests have been used to compare them with that of a multinational company. For this purpose, we conducted their physicochemical parameter’s evaluation, to get a stable and effective drug product which may be pharmaceutically equivalent to a multinational product. The safety and efficiency of these drugs was measured by uniformity content. The amount of cetirizine 2HCl in the different local brands available in Lahore, is proved to be within limits, so these can replace a multinational brand at a low cost.
The present study is based on spectrophotometric method for chemical assay, TLC technique for the purity of API’s and physiochemical parameters i.e. weight variation, hardness, friability, thickness and disintegration time as well as price fluctuation in PK rupees. Weight variation of the tablets were examined; all the tablets weight are in accordance with the required limit (NMT ±5%), hardness is tested; the hardness is in good agreement with the specification (5-10 kg/cm2), thickness and the friability of the tablets are determined which complied with the specification (3.5 mm and NMT 1% ) respectively; disintegration test is performed, all the tablets were disintegrated with the prescribed time ( NMT 30 minutes); TLC technique is developed to check the quality and purity of API, all the active ingredients showed the similar \(R_{f}\)-values without any impurity (0.38) ; pharmaceutical assay was carried out, none had potency less than the required specification ( 90% to 110% ).
From the comparative study, it is observed that all the brands of cetirizine 2HCl, tablets (10mg) showed the same results as per I.P standards .Since there is a variation in the formulation of these drugs which may affect the results by performing qualitative and quantitative tests. From the test results and plots, it is observed and concluded that an economical and quality products can be prescribed for allergy patients whether they are manufactured by the local or multinational pharmaceutical companies without any health risk.