We explore the possibility of using an iron-based anodic material (\(\alpha\)-hematite) synthesized with a hierarchical 3D urchin-like morphology, as an OER catalyst. The electrodes are prepared by pulsed laser deposition followed by thermal annealing leading to the hierarchical 3D urchin-like morphology. The effect of the deposition parameter on the catalyst phase and morphology are investigated by microRaman spectroscopy and scanning electron microscopy, while the electrode metrics are determined by voltammetric methods and Tafel analysis. We observe that the material is highly electroactive towards the OER, with performance in-line with that of noble-metal based state-of-the-art catalysts.
To reduce our dependence on fossil fuels and reduce the emission of carbon dioxide, a large scale transition toward sustainable energy sources is necessary [1]. In this regards Hydrogen could play an important role in our modern life. It is a promising energy carrier, which could have a low impact on the environment and its energy content is 10 times greater than fossil fuels. Hydrogen production through the water splitting is a cheapest and clean source of energy [2]. On large scale production of hydrogen from the water splitting, largely depend upon the catalysts that are require to overcome the challenging requirement thermodynamically and kinetics of this half reaction [3, 4]. From an electrochemical point of view, it can be split into the two half reactions:
The oxygen evolution reaction (OER) is a key reaction and often the rate-determining step in electrochemical and Photoelectrochemical water splitting, which is a promising route to carbon-free hydrogen production. For this process we require the OER catalysts which are close to the state- of art catalysts. Iron oxide (\(\alpha\)-hematite) because it has been identified as a suitable water oxidation catalyst [5, 6, 7, 8, 9, 10], conjugating an overall good performance in both electrolysis and solar water splitting schemes with the advantage of being earth-abundant, non-toxic and environmentally safe.
Pulsed-laser deposition (PLD) of thin films is a technique that employs high-energy-density laser pulses to generate, in the regime of phase explosion, ablated material from a solid target, consisting of a mixture of vapor/liquid nanodroplets. PLD could present some significant advantages over the methods listed above: precise control of the quantity of the deposited material, enhanced adhesion due to the energetic nature of the process, and, most importantly, the possibility of nanostructuring the surface by the deposition of nanoparticles (NPs) [11, 12]. Additionally, being essentially a physical deposition method, it is suitable to all kind of substrates. The main drawback of the PLD technique is the need of specialized equipment, although this is already employed in industrial applications.
The target for the Pulse laser deposition consisted of the cold pressed with a pressure of about \(450-500Kg/cm^2\) for two times consecutively powders of Fe and Boric acid. Two sets of electrodes, fabricated on the FTO substrates with different thickness by changing the number of pulses: 5000 pluses for the thinner samples and \(10000\) pulses for the thicker samples. The fluency of the laser was fixed at 3\(J/cm^2\) and the deposition was carried out in a reactive oxygen atmosphere, at a pressure of \(1.5\times10^{-2}\) mbar. The number of pulses set at \(5000\) and the repetition rate at \(20Hz\). The coatings were deposited on FTO substrates, with a target – substrate distance of \(4.5 cm\). All the sample were subjected to a post-deposition annealing treatment, carried out at different Temperature i.e. \(500^oC, 600^oC\) and \(700^oC\) for 4 hours with heating rate of \(5^oC/min\) and one sample subjected at \(600^oC\) for 4 hours and again at \(800^oC\) for 1hours with heating rate of \(5^oC/min\). We represent the differently annealed sample with the following symbols:
| Sample | Temperature |
|---|---|
| Thin sample annealed at \(500^oC\) for 4 hours | ANN\(@500^oC\) |
| Thin sample annealed at \(600^oC\) for 4 hours | ANN\(@600^oC\) |
| Thin sample annealed at \(700^oC\) for 4 hours | ANN\(@700^oC\) |
| Thin sample annealed at \(600^oC\) and \(800^oC\) for 4 hours | ANN\(@600^oC+800^oC\) |
| Thin sample annealed at \(500^oC\) for 4 hours | ANN\(@500^oC\) thick |
| Thin sample annealed at \(600^oC\) for 4 hours | ANN\(@600^oC\) thick |
| Thin sample annealed at \(700^oC\) for 4 hours | ANN\(@700^oC\) thick |
| Thin sample annealed at \(600^oC\) and \(800^oC\) for 4 hours | ANN\(@600^oC+800^oC\) thick |
The following SEM images show the morphology of the thin films. Samples ANN\(@500^oC\) Figure 1 and ANN\(@600^oC\) Figure 2 show the presence of urchin-like structures, increasing the surface of the catalyst and thus the amount of active sites for the water oxidation.

The lengths of needles are not homogeneous, dense and aggregated nanoparticles are found at \(600^oC\) annealing temperature. Furthermore, SEM images show the nano needle with a non-uniform morphology. Samples show spherical nanoparticles covered with sparse nano flowers. The flower consists of needles petals grown radially from the surface. Probably the roughness present on the core surface is the starting point for the formation of these features. Overall SEM images confirm the urchin like structure on samples ANN\(@500^oC\) and ANN\(@600^oC\).

Sample ANN\(@600^oC + 800^oC\) Figure 3, show that there is no developed urchin like structures. At \(700^oC\) Figure 4 samples show spherical nanoparticles covered with sparse nano flowers. The flower consists of needles petals grown radially from the surface. Probably the roughness present on the core surface is the starting point for the formation of these features. As will be shown in the following sections, the electrochemical performance of these samples is not good. Here I discuss the thin sample SEM images because these are similar to the thick sample.

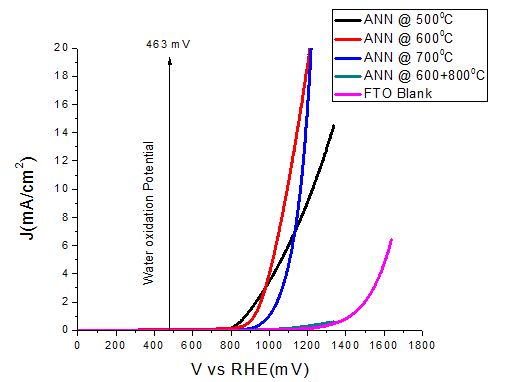
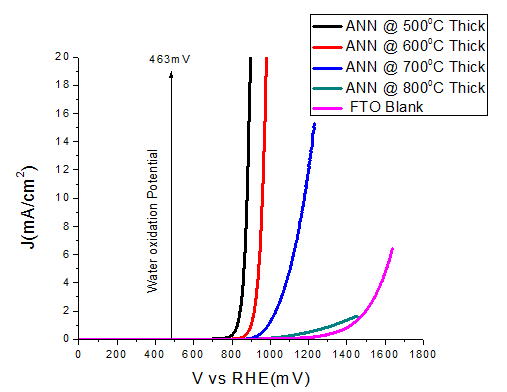
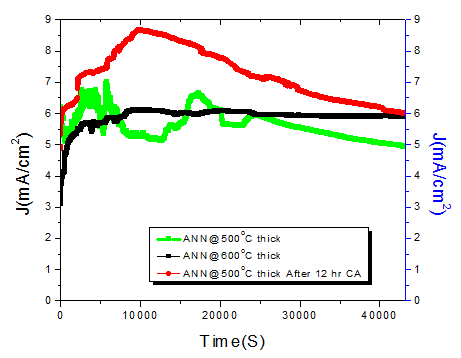
The samples ANN\(@500^{o}C\) and ANN\(@600^{o}C\) show good catalytic performance, in term of overpotential. Large overpotential is associated with slower kinetics for the OER on hematite and with the electronic and structural characteristic of the hematite/electrolyte and hematite/substrate interfaces. After increasing the thickness of the film, then again film ANN\(@500^oC\) thick and ANN\(@600^oC\) thick showed good catalytic performance, both in term of overpotential and tafel slope as compared to the thin films (Table 1). Other samples ANN\(@700^oC\) and ANN\(@600^oC\)+\(800^oC\) display the bad catalytic enactment. The detrimental in water oxidation activity for the samples annealed more than \(600^oC\) could be due to the losing of the conducting properties of FTO. Since, it is clear known that FTO losses its conductivity above \(550^oC\). Thereby, it is clear that the decreased activity is mainly due to the losing FTO conductivity at high temperature annealing.
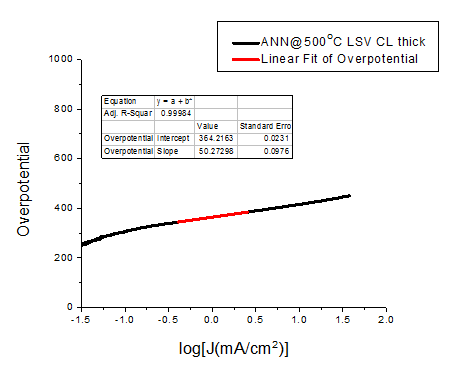
The overpotential is calculated as
$$Overpotential=V_{Applied}+0.241-E^oO_2/H_2O$$
Where \(E^oO_2/H_2O = 463\) mV at pH = 13 and the 240 mV term is required to convert the applied potential Vapplied from SCE to RHE (Reversible Hydrogen Electrode). Samples overpotential and Table slopes are shown in Table 1.
| Sample | Overpotenital | Overpotenital | Overpotenital | Tafel slope |
|---|---|---|---|---|
| at 0.2(mA) | at 1(mA) | at 10(mA) | mV/dec\(^{-1}\) | |
| Ann\(@500^o C\) thin | 343.9 | 412.4 | 769.8 | 81.6+0.6 |
| Ann\(@600^o C\) thin | 370.0 | 457.0 | 631.1 | 109.8+0.2 |
| Ann\(@700^o C\) thin | 455.6 | 534.5 | 699.9 | 135.8+0.5 |
| Ann\(@600^o C+800^o C\) thin | 708.9 | 103.9 | — | 373.7+2.9 |
| Ann\(@500^o C\) thin | 329.1 | 364.5 | 416.5 | 50.2+0.09 |
| Ann\(@600^o C\) thin | 398.8 | 436.6 | 495.6 | 56.0+0.1 |
| Ann\(@700^o C\) thin | 463.5 | 526.4 | 414.4 | 83.2+0.7 |
| Ann\(@600^o C+800^o C\) thin | 615.2 | 865.0 | — | 258.8+2.8 |

I am raw html block.
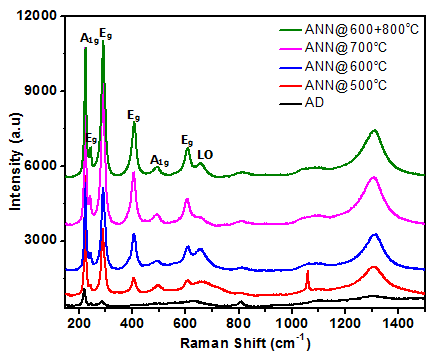
Click edit bBefore and after water oxidation the miro-Raman spectra for all the samples are represented in the Figure 9. It is indicating that after water oxidation the peak intensities were reduced, the probable reason could be due to the surface coverge of the electrolyte. However, the phase and crystallinity of the all the samples were well preserved ever after water oxidation, showing that the prepared thin film samples are highly stable to the water oxidation experiments.
