This review provides a comprehensive overview of the synthesis process of nanoscale materials and highlights key characterization methods used for nanomaterials and biomaterials. It emphasizes the importance of effective techniques for investigating materials at the nanoscale, as these are too small for the human eye to detect. The review also explores various approaches to producing nanoscale materials and offers insights into the application, development, advantages, and limitations of different experimental methods for nanoparticle characterization. A particular focus is placed on advanced characterization techniques and their role in data interpretation, aiming to guide novice researchers in the field of nanoscience and nanotechnology.
There are several approaches for the synthesis of materials at nanoscale. This review is intended to provide an overview of current understanding on the application, developments, benefits, and drawbacks of the several experimental methods accessible for nanoparticle characterization. The novice researcher in the field frequently lacks knowledge of the sophisticated characterization techniques and their applications for data interpretation. One of the fields experiencing constant advancement is material science, whose expansion is a mirror of civilization. For example, the stone era dates to around 5000 BC, the copper age to 5000–800 BC, the bronze age to 300–00 BC, the iron age to 800 BC–40 AD, and the plastic age since 1907, which coincided with the discovery of Bakelite [1]. As a result, we may observe that a specific time frame is known after the material we utilize. It is likely that gold was the first elemental metal that humans used [2]. Copper was the next metal that humans used after gold. Archeological research on the Indus Valley Civilization provides evidence for this. In order to achieve desired qualities, material scientists and engineers have recently concentrated on creating materials that are customized starting at the atomic level. The breakthrough developments in material science and engineering, along with their effects on society, have been referred to as the customized material era [3].
We need a variety of materials in different forms than those found in nature for today’s activities to run smoothly and comfortably. Man thus attempted to change the characteristics of materials according to necessity. Man engaged in a variety of processes, including mixing, alloying, heating, cooling, melting, and other processes, to impart qualities that differed from its native condition. The materials can be given the necessary and beneficial qualities through a number of small but crucial actions. [4]. Life is made easier by the advancement of several cutting-edge technologies. For instance, the majority of the materials used in cars and airplanes in the past were metallic. Those vehicles were very heavy because metals have higher density. As a result, reaching high speed proved challenging. As a result of technological breakthroughs, composite materials are replacing metallic parts in automobiles [5]. These vehicles are lightweight and capable of fast speeds.
The study of the connections between the composition, characteristics, and processing of materials is the focus of the scientific field of material science [6]. Material engineering is a branch of engineering that arises when material knowledge is used to create a technical product. One of the most significant technical achievements of the past century has been considered to be the advancement of sophisticated materials. Material pertaining to nanotechnology and nanoscience is given special attention in the state-of-the-art research activities. Engineering materials must have a number of attributes in addition to the primary ones, including stability, durability, resistance to chemicals, corrosion, wear, impact, heat, and shock. Any region needs some encouragement in order to grow and thrive. Therefore, the following are motivating elements for the advancement of nanoscience and technology:
i. They differ from bulk materials in that they display intriguing behaviors at the nanoscale size scale, and
ii. These qualities can be applied to technologies (particularly those related to health, known as bio-inspired nanotechnology) that promote human prosperity.
The main driving force behind the fields of nanoscience and nanotechnology has been the desire to replicate how materials are synthesized and manipulated at comparable length scales in nature. Scientists have been motivated to create new nanomaterials with exact control over their shape and size by the intriguing characteristics and alluring architectures of biomaterials [7]. These nanoscale materials have unique properties that rely on size and shape, making them valuable for a wide range of scientific and practical applications. As a result, new technological advancements are enabling the creation of structures or devices smaller than 100 nanometers that have significant functional benefits over traditional devices, bringing them closer to the point of revolution. It has the power to completely alter the current technological landscape. Indeed, the claims are so lofty that they can even meet the Millennium Goal of providing all people with affordable facilities. Applications of nanotechnology are becoming more widespread in a variety of items, including food, electronics, automobiles, apparel, and many biological fields [8– 11].
The bulk of research is being conducted in the realms of nanoscience and technology, if we peep into scientific disciplines like physics and chemistry [12]. The Greek word Nano means small. We are aware that for small objectives we use word Nano for example Nano car. However, according to science, a material is considered to be in the Nano domain if it has at least one feature that differs from that of the bulk and falls between 1 and 100 nm in any dimension. It goes without saying that extremely small systems and objects are the focus of nanotechnology, nanoscience, and Nano-engineering. Nanoscale structures are thought to lie on the boundary between the largest molecule in a living system and the smallest man-made technology. Exploiting novel physical, biological, and chemical features of systems that are in between single atoms and bulk materials will be made possible by our ability to control and change nanostructure. Currently, the entire range of operations leading to the anticipated next industrial revolution is comprised of nanotechnology and the related research field.
Various scientists have given different definitions to nanotechnology. According to Hunt, nanomaterials constitute an enabling component of the well-known field of nanotechnology [13]. Nanotechnology, according to Scientific Americana, is “a vast grab bag of stuff that has to do with creating tiny things that sometimes just happen to be useful.” It contains extensive references to condensed matter physics, engineering, chemistry, and molecular biology [14]. However, the National Nanotechnology Initiative (NNI) of the United States offers a more comprehensive definition, which is as follows: (i) atomic, molecular, or macromolecular research and technology development; (ii) the creation and use of structures, devices, and systems that have special qualities and functions because of their small and intermediate size; and (iii) the ability to control or function at the atomic level, with a length of approximately 1 to 100 nanometres [15]. The Royal Society and the Royal Academy of Engineering also describe it as “the design, characterisation, production, and application of structures, devices, and systems by controlling shape and size at the Nano-meter scale [16]”. Consequently, improving material properties by manipulating their nanoscale structures forms the basis of nanotechnology.
Materials can be classified at the nanoscale in several important and well-known ways according on their dimensionality. Let’s examine the three-dimensional space vectors of a specific nanomaterial. If the diameters of nanomaterials lie inside the critical range of 1-100 nm in all three dimension vectors, they can be referred to as 0-dimensional particles or quantum dots, such as spherical nanoparticles of Au, Ag, CdS, CdSe, etc. The dimensionality of quantum dots is actually quite small, ranging from 1 nm to 5 nm. When a nanomaterial may only grow in the third direction within a crucial range of 1–100 nm in two spatially dimensional vectors, it is referred to as a 1D nanostructure. Examples of such nanostructures are single-walled carbon nanotubes, quantum wires, and nano-rods. Nanomaterials can grow in two-dimensional vectors, such as quantum sheets, in 2D nanostructures, where only one dimension is constrained to that critical regime. Figure 1 diagrammatically illustrates this idea.

In a similar vein, core-shell nanoparticles are a separate family of nanomaterials whose surface and core regions have different chemical compositions [17]. The intriguing electrical behavior of non-metallic nanoparticles, which are made up of organic and non-metal molecules, may be adjusted to be conducting or insulating depending on their size and makeup. Fullerenes, which are used in super-conductors and medicine, are the most well-known type of nonmetallic nanoparticle [18]. Another well-known family of nanomaterials are carbon nanotubes, which, depending on their diameter and chirality, can be either metallic or semiconducting.
Since we know that materials at the nanoscale have different properties from those in bulk, these qualities can be applied to a wide range of engineering applications. For greater strength at high temperatures and resistance to corrosion, researchers are continuously investigating new and enhanced aircraft alloys, such as nickel-base super alloys. The alloys are utilized in engine parts, and high operating temperatures can result in improved engine efficiency. In order to create novel materials with unique features, nanoscale materials research is ongoing. Materials made of polymers have been the material with the quickest rate of growth worldwide in recent years, and they have replaced packaging papers, glass, and metals [19]. Nanotechnology has also been used to create better ceramic materials that meet the needs of engineering applications with high temperatures and high wear conditions [20]. In recent years, smart materials based on nanotechnology have emerged for application in sensors and actuators.
A new vocabulary the term “smart material” refers to a substance that reacts well to variations in physical parameters like temperature, pH, moisture content, or electromagnetic fields. Smart materials may change their size and shape just by applying a small amount of heat. Additionally, when placed close to a magnet, they can virtually immediately transform from a liquid to a solid [21]. One or more of the properties of smart materials can be significantly altered. Nowadays, material scientists are working hard to create novel materials by nanotechnological means. Materials utilized in biochemical analysis and robotics also belong to the category of smart materials [22]. Many kinds of smart materials are being developed, such as digital blood pressure monitors, blood glucose sensors etc. The machine makes an odd sound to signal that something is wrong with the patient when the blood pressure level rises above the critical level. Nowadays, researchers from a wide range of disciplines, including physics, chemistry, mechanical engineering, microbiology, and the medical sciences, rely heavily on nanotechnology, which has become a buzzword. In actuality, nanotechnology has blurred the boundaries between scientific fields. This is due to the fact that the nanoparticles are not believed to follow the classical but adheres to quantum mechanics [23]. Every substance maintains its characteristics down to the micro level [24]. However, a material’s characteristics drastically alter once its size is lowered below the micro level. “Every great advancement in science has emerged from a new audacity of imagination,” according to John Dewey in his Quest for Certainty [25]. The term “nano-scale” thus conjures up the world of the tiny, in which a nanometer is equivalent to a billionth of a meter. Therefore, the question of whether to use the terms nanometer, 10nm, 100nm, or 1000nm would be evident. The response to this Although this is up for debate, the majority of scientists believe that particles with at least one feature that differs from their bulk form and that range in size from 1 nm to 100 nm in any dimension are considered nanoparticles. Figure 2 illustrates how a few nanomaterials’ sizes can be practically understood by contrasting them with substances or species that occur in the biological world.

The Father of Nanotechnology, Richard Feynman, was the first visionary to draw attention to this possibility. He stated in his well-known address on December 29, 1959, “There is plenty of room at the bottom.” This has given rise to a new field of study that is currently called nanoscience and technology, in which everything is dependent on our capacity to create and manage materials at the nanoscale, atom by atom and molecule by molecule. Norio Taniguchi, a Japanese physicist, first used the word “nanotechnology” in 1974 [26]. According to his assertion, “Nanotechnology mainly consists of the process of A single atom or molecule has the ability to detach, solidify, and distort materials. Nanomaterials’ special size and shape-dependent properties make them useful for a range of scientific and technological applications [27].
Nanoparticles have been used for thousands of years, with early references found in the ancient Indian medical system of Ayurveda, where techniques for creating metallic nanoparticles, known as bhasmas, were detailed [28– 32]. This practice, described in texts like the Ras Ratnakar by Nagarjun, involved purifying and transforming metals for medicinal use. Similarly, Dr. Samuel Hahnemann’s homeopathic treatments also utilized metallic nanoparticles, such as nano silver and nano gold, for healing purposes. The use of nanoparticles continued throughout history, including alchemists’ creation of “potable gold” and “potable silver” in the 16th century, and their optical properties were harnessed in artifacts like the Lycurgus cup, dating back to the 4th century AD. The science of nanostructured materials began to take shape in the 17th century with the study of stained glass windows, and Faraday’s work on gold nanoparticles furthered the understanding of their unique characteristics [33– 35].
In recent decades, advances in electron microscopy have sparked a renewed interest in nanoscale materials. This has led to a boom in nanotechnology, with applications ranging from biosensors to medicine. As Nobel Laureate Richard Smalley predicted, the future of nanotechnology promises to transform industries and everyday life, as materials at the nanoscale exhibit properties influenced by factors such as size, shape, and surface composition [36-38]. The historical development in the domain of domain of Nanoscience and technology is given as below in Table 1.
| 5000 years ago | Ayurveda system of medicine developed from Atharva-veda describes use of nanoparticles in medicine |
| 4th century | Lycurgus cup |
| 500-1450 | Cathedrals stained with glass window |
| 2000 years ago | Sulfide Nanoparticles were used by Greek and Romans to dye the hair. Ancient Indian cave paintings contained nano-materials. |
| 1000 years ago | Gold nanoparticles of different size and shapes were used to color the glass |
| 1857 | Michel Faraday synthesized colloidal solution of gold |
| 1905 | Einstein publishes his study on the dimension of sugar molecule, approximately 1 nm. |
| 1908 | Gustav Mie light scattering nanoparticles |
| 1928 | Edward Synge Near field optical microscopy |
| 1931 | Knoll and Ruska Electron microscope |
| 1936 | Invention of field electron microscope |
| 1947 | John Bardeen Discovery of semiconductor |
| 1950 | Victor La Mer and Robert Dinegar developed theory and process of growing nanoparticles |
| 1951 | Erwin Muller invention of field ion microscope |
| 1956 | Molecular engineering term was coined by Arthur von Hippel which is applied to dielectrics, ferroelectrics and piezoelectrics |
| 1958 | Concept of First Integrated circuit was developed |
| 29th December 1959 | R. Feynman, in his lecture at the annual meeting of American Association of |
| Physical Sciences, claims that There is Plenty of Room at the Bottom | |
| 1965 | Moore’s law was developed |
| 1974 | Norio Taniguchi introduced the term nanotechnology |
It has been observed that the properties of the materials vary from its bulk counterparts once they are below millimeter dimensions. The unique characteristics of the materials are many times useful in scientific and technological developments. The optical, mechanical, electrical properties of the material changes at nanoscale. Therefore, researchers from various discipline of science and technology are working in the area to design and characterize materials at nanoscale and see its applications in their own field. [39, 40]. It has been already stated that the properties of the material changes at nanoscale. It is worth to mention here that not only the properties merely depend upon size and shape, but it depends upon other factors also such as nature of stabilizing agent, production route, nature of reducing agents etc. At the nanoscale, for instance, variations in optical, thermal, electrical, electronic, magnetic, and mechanical characteristics are discernible. These characteristics are briefly discussed below.
The physical properties of the materials vary at nanoscale. It has been observed that melting point of nanomaterials are lower than its bulk counterparts. The reduced melting point can be graphically represented as shown in Figure 3.

It is evident from the melting point (Tm) vs. particle size (D) graph above that the melting temperature drops as particle size decreases. The melting point drastically drops below 3–4 nm at the nanoscale. The following is a description of the cause of the nanoparticles’ decreased melting point: Because the surface atoms in the solid phase are coordinatively unsaturated, they have a high surface energy. The liquid phase always has a lower surface energy than the solid phase. Because the melting process begins at the surface, the system of nanoparticles is significantly more stable in the liquid phase because of the lower surface energy. The melting point of the nanoparticles drastically drops below 3–4 nm.
A number of factors, including surface to volume ratio, impurity, and dislocation, affect the material’s mechanical strength [41]. As the number of material flaws increases, the mechanical strength falls. Grain size affects the material’s hardness and strength. Nanomaterials such as carbon nanotubes, nanowire, or nanorods have better mechanical strength because of their narrow cross section and low number of flaws. Super-plasticity, on the other hand, is the well-documented ability of nanomaterials to experience significant tensile deformation without losing their structural integrity [42]. Because of their high surface to volume ratio, nanoparticles create distinct local environments for their surface atoms. The material’s mechanical strength is determined by a number of factors, including surface to volume ratio, impurity, and dislocation [41]. The more material flaws there are, the lower the mechanical strength. Grain size determines the material’s strength and hardness. Materials with a narrow cross section and fewer flaws, such as carbon nanotubes, nanowire, or nanorods, have better mechanical strength. Nonetheless, super-plasticity—the ability of nanomaterials to experience significant tensile deformation without losing their structural integrity—has been widely documented [43]. Different local environments are created for the surface atoms as a result of the nanoparticles’ high surface to volume ratio.
In particular, optical characteristics have become essential for studying noble metallic nanostructures. This is because they display the phenomenon of surface Plasmon resonance and an absorption profile that is dependent on size, shape, composition, and the surrounding medium [44]. The optical characteristics of metal nanoparticles differ from those of their bulk counterparts. The size affects the optical characteristics. In the form of its colloidal solution, gold, a yellowish metal, can be obtained in a variety of hues. The way the nanostructure interacts with light is what gives the butterfly its distinct hue. Figure 4 depicts the size-dependent optical characteristics of materials at nanoscale. As the nanomaterials in solution varies in their size and shape, it shows multiple color.

The incident electromagnetic radiation generally in the UV and Visible range, interacts with the electron cloud present on the surface of the materials. This interaction results in the phenomenon known as Surface Plasmon Resonance. This phenomenon can be defined as,’ Collective oscillation of surface electrons due to interaction with electromagnetic radiations.’ When the frequency of photons equals the natural frequency of surface electrons oscillating against the restoring force of positive nuclei, the resonance condition is met [45].
The formation of electron cloud is the result of accumulation of atoms on the surface of materials. As we are aware that electrons are released from the metals due to electropositive nature of metals. The released electrons are accumulated and the positively charged cations are supposed to be embedded into the sea of electrons. These free electrons can be move under the influence of electromagnetic radiations as shown below in Figure 5 [46, 47].

This causes the surface electrons, whose thickness is about equal to the screening length of a few angstroms, to oscillate while the electron density inside the particle remains constant. Mie first used Maxwell’s equation of scattering to describe surface Plasmon resonance, a unique property of nanoparticles, in 1908 [48]. The optical constant of the bulk metal can be used to compute the particle’s absorption spectrum in a particular solvent. For spherical particles with diameters ranging from 3 to 20 nm, the absorption spectra are not significantly influenced by particle size. This is because the particles are smaller than the size at which the absorption constant’s upper order elements in the Mie formula become important, therefore only the dipole terms need to be taken into consideration. These terms rely on the total metal concentration in the solution rather than particle size [49]. As particle size increases, higher order modes take center stage, red shifting the Plasmon absorption band and expanding the bandwidth. Higher order modes are excited when the particle size is large because the light cannot uniformly polarize the nanoparticles due to retardation effects [50]. The dipolar approximation can be used to address nanoparticles within the 25 nm size range, and the absorption profile is not significantly influenced by particle size [50– 52]. Further, it has been observed that the particles exhibit size dependent Surface Plasmon Resonance in between 2-10 nm size range.
There are two ways in which the magnetic properties of noble metallic nanoparticles differ from those of bulk. The high surface to volume ratio, which creates distinct local environments for the surface atoms in their magnetic coupling/interaction with nearby atoms, is one of the causes of the aforementioned feature [53]. Several small ferromagnetic particles may consist of only one magnetic domain, in contrast to bulk ferromagnetic materials that often form several magnetic domains [54]. A single particle exhibits super magnetism when its magnetization is dispersed randomly and only aligned when a magnetic field is introduced; when the magnetic field is removed, the alignment is no longer present [55]. Super-magnets, optical recording or storage media, and other commercial and research applications use this effect. Due to their numerous applications in fields like catalysis, biomedicine, magnetic resonance imaging, magnetic particle imaging, data storage, environmental cleanup, optical filters, etc., the magnetic characteristics have recently been the subject of a lot of research. [56].
It is well recognized that nanoparticles are crucial to numerous biological and pharmacological processes. In the study of nanoscience and nanotechnology, it is crucial to consider how nanoparticles impact the cytotoxicity levels in living systems [57]. Numerous biological applications as well as other sectors have made use of nanoparticles [58]. The fields of bio diagnostics, therapeutics, drug delivery, bioimaging, immunostaining, and biosensing have all shown promise for the future. Studying the short- and long-term impacts of size, shape, and surface functional groups on these various nanostructures’ bioavailability, subcellular distribution, metabolism, and degradation becomes crucial [59].
The materials exhibit a variety of electrical characteristics at the nanoscale. At the nanoscale, metals with a reputation for high conductivity act like semiconductors [60]. As previously mentioned, the characteristics of samples in the nanoscale range can be significantly impacted by their size. Electrons are restricted to a small radius at the nanoscale. One of the most amazing phenomena that quantum dots can produce is single electron tunneling. A single additional electron added as a result of one electron tunneling can significantly alter a dot’s capacitance. Researchers are working to create translucent, flexible coverings with exceptional electrical conductivity.
The percentage of surface atoms rises dramatically with decreasing particle size; for instance, a particle of 3 nm would have 45% of its atoms on its surface, while a particle of 1 nanometer would have 76% of its atoms on its surface. Small particle-sized nanomaterials with a high fraction of surface atoms make good catalysts since all reactions occur at the surface [61]. Although gold is regarded as a noble metal in its bulk form, gold nanoparticles dissolved in iron oxide or alumina have been shown to be highly effective catalysts for the oxidation of carbon monoxide. In addition to particle size, other parameters that affect the pace of chemical reactions include the nanomaterial’s form, surface structure, atoms’ electronic states, and the work function of a nanoparticle, among others [62]. The molecules of the reactant exhibit varying adsorption affinities for the various catalyst faces. Therefore, a catalyst’s selectivity could be improved by using metal nanoparticles with varying shapes and faces [63]. Therefore, surface reactivity that is customized by changing the nanoparticle’s shape may aid in the creation of molecularly specific catalysts.
The stronger confinement of electronic motion (spatial confinement) occurs as the particle size reaches the nanometer level because electronic motion is constrained to a smaller area than the mean free path of electrons. The quantum effect in these materials deviates from the classical theory of electronic motion. The valence and conduction bands are no longer inseparable when metallic nanoparticles’ electrical mobility is quantized, which confines them to specific discrete energy levels. At a certain size range, the Kubo gap—the energy gap between the valence band and the conduction bond—becomes equal to or greater than thermal energy (KBT), making metallic nanoparticles semiconductors. As the size of the particles decreases, the confinement increases and the material eventually turns into an insulator [64]. At this point, the material exhibits characteristics not possible from its bulk or individual equivalents and behaves differently in response to varied stimuli. Due to the execution of electronic motion in diverse dimensions, nanomaterials demonstrate intriguing shape dependence in addition to the size dependence of their various features. For instance, 0-D nanostructures exhibit the electronic tunneling phenomena, which is a fundamental idea in the construction of artificial atoms and devices such as single electron transistors [65]. Similar to this, an electron in a one-dimensional nanostructure can oscillate in both longitudinal and transverse modes when exposed to an electromagnetic field. Because of the two distinct forms of electronic mobility, nanorods and nanotubes produce Surface Plasmon absorption peaks. The way electrons move also changes their other features.
As discussed earlier the change in properties at nanoscale is attributed to the following reasons.
Nanoparticles are having high aspect ratio i.e. surface to volume ratio. For example, for a spherical particle, surface area = and volume = 4/3 \(\pi\) r3. The surface to volume ratio viz. size of the particle is shown below in Figure 6.

Atoms coordinate with their environment. All of the atoms in the bulk material can complete their coordination number with their surroundings, but atoms on the surface cannot. The surface becomes more reactive as a result of the surface atom not completing its valence and the empty bond, known as a dangling bond. As a result, the nanoparticles are more unstable than their bulk counterparts.
The size and separation between objects are determined by the strength of the forces (gravitational, electrostatic, etc.) between them. Gravitational forces are so weak at the nanoscale that they can be disregarded [66]. This is comparable to escape velocity, which is the point at which gravity is no longer effective at a very high speed of 11.3 km/s. Despite its strength, the nuclear force might be disregarded due to its narrow range [67]. A number of electrostatic forces, such as the Van der Walls force, are particularly significant at the nanoscale. They play a particularly significant role in self-assembly. The creation of novel techniques for the synthesis of noble metallic nanoparticles in various media with variable size, shape, and long-term stability is the primary goal of this thesis. The techniques for creating metal nanoparticles and their stability are covered in the next section.
Although nanoparticles are naturally unstable and have a propensity to aggregate, stable nanoparticles are necessary for all applications [68]. Gold nanoparticles have been functionalized using a monolayer or mixed monolayer of distinct molecules with varying functionalities at both ends. These techniques used weak reducing agents to reduce metal ions when capping molecules were present [69]. The stoichiometric ratio of metal ion to capping ligand concentration determines the size of the nanoparticles during production. With the aid of ligand exchange processes, it can be further altered to produce other functional compounds. Surface-modified nanoparticles frequently lose stability as a result of strong intermolecular interactions between capping agents and the ionic strength of fluids.
New capping molecules with distinct amino acid sequences that imitate the properties of naturally occurring non-aggregating proteins have been created in order to solve this issue. Even in the presence of salt in the aqueous phase, these capping ligands easily adhere to the surface and create a densely packed passivating surface with a hydrophilic terminal, making it soluble and stable. Stabilizing nanoparticles can be accomplished by manipulating their surface characteristics [70]. Numerous applications, including medicine delivery systems, electronic devices, and solution-based sensors, have demonstrated the use of nanoparticles. Two benefits of surface modification are that it prevents agglomeration and gives the surface of the nanoparticles a variety of functions.
For stability and intended functioning, surface modification of nanoparticles is crucial. Compared to bulk atoms, surface atoms have a lower coordination number. As a result, it feels pressure from within and strives for the highest coordination number. Compared to bulk atoms, it produces a shorter bond length with its immediate atomic layer. The bond length with the subsequent layer of atoms diminishes as the nanoparticle size lowers because an increasing number of atoms are exposed to the surface. Because there are so many atoms on the surface at such a small scale, the lattice properties of the nanoparticles change. Energy required to bring back these atoms to its original position is nothing but surface energy [71]. As the particle size of the nanoparticles decreases their surface energy increases significantly. Increase in the surface energy also results in the increase in the Gibb’s free energy. According to the law of thermodynamics, every system always tries to attain minimum Gibb’s free energy; therefore, it loses its nanoness and exotic properties related to it. Hence it is very important to stabilize the nanoparticles against the aggregation. There are many ways by which nanoparticles can be stabilized [72].
Particularly during the production of noble metallic nanoparticles, the adsorption of reactant ions on the nanoparticle surface results in the formation of an electronic double layer around the particles. The Van der Waals forces of attraction and the electrostatic force of repulsion caused by the charged ions on the surface are two forces operating on the nanoparticles at the same time. The interaction of these two forces determines the stability of nanoparticles. The electrostatic stabilization of nanomaterial is shown below in Figure 7.

The potential energy versus distance graph between the nanoparticles is displayed in Figure 7. Van der Waals forces of attraction and electrostatic repulsion are zero at great distances. Because of the strong dominating Van der Waals forces of attraction, the potential energy curve has a deep minimum at zero distance. The potential energy diagram’s maxima are the consequence of electrostatic force overriding the force of attraction as nanoparticles move apart. The repulsion energy barrier between two nanoparticles is this maximum. The thickness of the double layer that forms around the nanoparticles determines the potential barrier. The potential energy barrier increases with double layer thickness [73]. The solution of nanoparticles stays stable if this barrier is higher than a specific value. The kinetic stabilization method known as electrostatic stabilization works best in diluted solutions. Aggregation results from the double layer charge being screened by the addition of electrolytes [74].
These drawbacks can be addressed by capping nanoparticles with appropriate ligands. As was previously indicated, two- and three-dimensional nanostructures can be synthesized using the lithographic process. However, the two-dimensionality of the lithographic stage is an intrinsic restriction of these approaches. The ability to manipulate the interactions surrounding a nanomaterial’s surface allows for the construction of three-dimensional structures. Additionally, capping compounds reduce the nanoparticles’ surface energy and stop their unchecked development [75].
Since small particles are unstable and prefer to aggregate to create bulk in order to obtain stability, stability is always a significant concern when metal nanoparticles are produced in solvent. The repulsive double layer and steric interactions would counteract the aggregation that would result from two particles being attracted to one another at a short distance. When a nanoparticle is created in an aqueous medium, the precursor ions that surround the nanoparticles adsorb, creating an aqueous double layer. Columbic repulsion between particles occurs when counterions instantly encircle this layer of ions. Because of their charge, nanoparticles are unable to aggregate. When there is low ionic strength and moderate surface potential in a solution, the electrostatic repulsion between the particles is enough to keep the particles from aggregating due to attraction forces [76]. The biomolecules used for stabilization of nanomaterials is shown below in Figure 8.

A variety of electron-rich ligands, including amines, thiols, phosphates, and carboxylates, have been employed to cap nanoparticles, as illustrated in Figure 8. With the aid of various capping agents, the nanoparticles’ surface charge, reactivity, specific gravity, and surface nature—that is, hydrophobicity—can be readily generated. The surface of nanoparticles has been altered using a variety of techniques, such as the coupling of biomolecules, such as proteins and amino acids, with monolayers and the mixed monolayer protection of molecules to metal clusters.
The stability of nanoparticles is derived from steric interactions through the physical or chemical adsorption of amphiphilic molecules, while electrostatic effects are less important when the particles are dispersed in an organic medium [77]. As seen in Figure 9, the lead group of these molecules attaches to the surface of metal nanoparticles while the hydrocarbon chain sterically inhibits aggregation. Even after the solvent has completely evaporated, the nanoparticles remain stable in the form of powder because of these steric interactions.

To alter the surface of nanoparticles, biomolecules like proteins, enzymes, and oligonucleotides have been employed. Utilizing the selectivity and reactivity of nanoparticles for various biological applications is the primary concept behind the coupling of big biomolecules with them [78]. In these situations, stabilizing agents that are readily interchangeable with biomolecules tend to minimize metal nanoparticles [79]. The surface of nanoparticles can be modified using this technique by adding various proteins, enzymes, thiolated DNA, etc. Biomolecules frequently link via electrostatic forces when nanoparticles are stabilized with anionic ligands like citrate [80]. The opposing charges on the nanoparticles and proteins enable electrostatic interaction.
The employment of other capping agents, such as thiols and phosphates, with ligands that have carboxyl, amino, or maleimide as terminal groups, is another strategy, as illustrated in figure 1.10. Through straightforward chemical processes like amidation, carbo-amide-mediated esterification, or interactions with thiol groups, these terminal groups can be utilized to couple nanoparticles with biomolecules. Such surface modification of nanoparticles can be used to increase their environmental friendliness and biocompatibility [81]. Steric interactions provide stability for nanoparticles dispersed in organic media, where electrostatic forces are less efficient. On the surface of nanoparticles, macromolecules such as polymers, surfactants, or ligands themselves adsorb. Figure 10 illustrates various thiols used for the generation of monolayer-protected gold nanoparticles.

In addition to these tactics, silica and polymers have been studied as capping agents [82]. Nevertheless, it is evident from the explanation above that surface modification with the right ligand is better than electrostatic stabilization for the reasons listed below: i) It provides thermodynamic stability
ii) It may be applied to multiphase systems
iii) It is not electrolyte sensitive and can be stored at high concentrations without aggregating.
The creation of innovative techniques for the synthesis of noble metallic nanoparticles in various media is the primary goal of this thesis. The techniques for creating metal nanoparticles and their stability are the primary topics of the section that follows.
For nucleation to take place in the instance of nanoparticle formation, the solution needs to be supersaturated. This can be achieved by adding the required reactant during the reaction or by dissolving the solute at a higher temperature and cooling it at a lower temperature. Nucleation and particle growth are the next steps in the precipitation process. Nucleation and growth typically take place simultaneously during particle formation, resulting in a final product with a wide range of sizes. Separating nucleation from growth is required to produce monodisperse particles. Because of their extremely high aspect ratio, nanoparticles are tiny and not thermodynamically stable. If the surface is not shielded, they attempt to merge with other particles, resulting in bulk structure [83]. When compared to materials made up of macroscopic things, nanomaterials are invariably thermodynamically unstable. As a result, they can be created at low enough temperatures to allow for kinetic rather than thermodynamic control over their growth.
As seen in Figure 11, the processes used to create nanoparticles can often be either top-down or bottom-up. The synthesis of nanoparticles was done primarily via a top-down method in the past [84]. The bulk metals are mechanically chopped down in the top-down method, and the resultant particles are then stabilized by colloidal protective agents. Numerous physical and chemical processes are used to reduce size. Metal vapor deposition techniques are one instance of a top-down laboratory synthesis method.
However, there are several disadvantages to this approach, such as the difficulty of achieving a narrow particle size distribution and the high installation requirements of metal vapor machines. The massive energy consumption required to sustain the high temperature and pressure employed in the synthesis process is another disadvantage of physical techniques. On the other hand, the majority of bioprocesses save a significant amount of energy because they operate at normal temperatures and air pressures [85]. Because the surface structure has a significant impact on the surface chemistry and other physical characteristics of nanoparticles, top-down production techniques produce flaws in the product’s surface structure, which is its main drawback. Table 2 shows distinguishing features between Top down and Bottom up approach.
| Feature | Top-Down Approach | Bottom-Up Approach |
| Definition | Involves breaking down larger structures into smaller nanoscale particles. | Involves building nanomaterials from atomic or molecular level. |
| Process Type | Subtractive (large pieces are broken down). | Additive (atoms/molecules are assembled). |
| Size Control | Size control is less precise due to physical fragmentation. | Size control is more precise as it constructs at the atomic/molecular scale. |
| Examples | Lithography, ball milling, etching, grinding. | Chemical vapor deposition (CVD), sol-gel process, molecular beam epitaxy. |
| Material Waste | Typically produces more waste due to fragmentation. | Generates less waste as it builds material from basic units. |
| Complexity | Generally simpler, involves mechanical or physical methods. | More complex, requires precise control over chemical reactions. |
| Scalability | Easier to scale up for large-scale production. | More challenging to scale up for mass production. |
| Applications | Used for producing materials with less precision, like coatings, and thin films. | Used for high-precision applications like nanostructures and devices. |
| Cost | Usually cheaper due to simpler processes. | Often more expensive due to complex equipment and processes. |
| Examples of Materials | Microparticles, thin films, nanostructured surfaces. | Nanotubes, quantum dots, nanoparticles, nanowires. |

These days, a bottom-up technique is typically used to create metallic nanoparticles. In the bottom-up method, metallic ions are reduced to metal in a condition of zero oxidation. Using different stabilizers or surfactants helps prevent the undesirable phenomena of clusters or aggregates. The basic idea is to create atoms in the solution that will instantly collapse into nanoparticles, then use a surfactant to control their final size and shape. The study of nanoscience in the twenty-first century has only recently begun in material science. Globally, research and development in this area is expanding quickly. There is now a lot of study being done on the physical characteristics of metallic particles at the nanoscale. Because of surface or quantum size effects, these species at the boundary between the molecular and solid states have unique physical characteristics.
Surface chemistry is of great importance in understanding the chemical and physical properties of nanoparticles [86]. For instance, the surfactant which forms a covalent bonding to metal nanoparticles can give an enhanced stability to nanoparticles. Understanding the interaction between surfactant and nanoparticle is critical and essential in understanding synthesis and application of nanoparticles [87]. There are almost no properties which do not change across the surface but none of them jumps truly discontinuously, rather they all change smoothly or in an oscillatory manner over a distance of a few atomic diameters. Atoms at the surface, edges, kinks and corners of crystallites are of higher energy than those in bulk. Their coordination number appropriately expresses the degree of unsaturation of their bonds [88].
In bottom up approach nanoparticles are built from smaller entities, for example by joining atoms, molecules and smaller particles. In this method of synthesis, the atomic particles are formed first and then assemble to form the final nanoparticle. The bottom up synthesis mostly relies on chemical and biological methods of production [89]. The traditional and most widely used methods for synthesis of metallic nanoparticles use wet chemical process [90]. A typical process involves growing nanoparticles in liquid medium containing various reactants, in particular reducing agents (e.g. sodium borohydride or potassium bitartarate or methoxy polyethylene glycol or hydrazine). To prevent the agglomeration of metallic nanoparticles, a stabilizing agent such as sodium dodecyl sulphate or polyvinyl pyrrolidone is also added to their reaction mixture [91]. Chemical processes are often inexpensive for large quantities, but they have drawbacks such as the production of hazardous byproducts, the use of toxic solvents, and contamination from precursor compounds.
As shown in Figure 12 various physical and chemical methods are extensively used to produce monodispersed nanoparticles, their stability and use of toxic chemicals in synthesis is of paramount importance. The use of toxic chemicals on the surface of nanoparticles and non-polar solvents in the synthesis procedure limits its application in clinical fields. Therefore, development of clean, biocompatible, non-toxic safe, cost effective, sustainable and eco-friendly methods for synthesis of nanoparticles is highly desired.
However, there are certain disadvantages to using microbes in biological techniques of nanoparticle synthesis, such as the time-consuming nature of microbe growth and the inability to better regulate the size distribution, shape, and structure of the nanoparticles [92]. Similarly, the sluggish production rate and non-monodispersed nature of the nanoparticles produced were drawbacks of the early biological approaches for nanoparticle synthesis. These are the issues that have beset methods of biological synthesis.

In order to provide ecologically friendly and biosynthetic technology for the production of nanomaterials, bio-nanotechnology—a fusion of biotechnology and nanotechnology—has arisen. Actually, scientists were aware of plant extract’s capacity to lower transition metal ions prior to the advancement of nanoscience and technology. Since the turn of the century, it has been recognized that plant extracts can lower metal ions. Plant extracts are environmentally benign materials that have recently been extensively studied in the production of silver nanoparticles. Certain dangerous compounds, such as sodium borohydride and hydrazine, can be substituted with plant parts that contain sugar or antioxidants throughout the manufacturing process. The presence of reducing and capping agents in the plant extract allows it to function so well in the creation of nanoparticles. Numerous scientists have created nanoparticles using different plant extracts. For the creation of nanoparticles, this technique eliminates the need for surfactants, capping agents, or templates.
Nature has been a source in medicinal plants for more than a thousand years, from the evolution of mankind. Over the last two decades, the need for medicinal plants has grown vigorously due to their herbal benefits as natural cosmetics and self-medication by public and biological effects. Due to increased urbanization, population explosion, and industrialization earth’s atmospheric condition has deteriorated. Over 80% world’s population uses plant-based medicines for primary health care. The plant produces a wide variety of secondary metabolites, which have multiple functions throughout the plant’s life cycle. Plant’s secondary metabolites are responsible for various functions such as infertility and germination of pollen grains. Numbers of plant’s secondary metabolites are used in food additives, fragrances, pigments, or directly in medicine.
Numerous flavonoids are significant medications. The reduction of silver and chloroaurate ions is thought to have been primarily caused by water-soluble heterocyclic compounds and polyol components found in leaves. Plant extracts are typically utilized directly during the manufacture of nanoparticles, without the separation of secondary compounds. However, a number of techniques, including Soxhlet extraction, heat-flux extraction, ultrasonic-assisted extraction, and microwave-assisted extraction, can be used to separate the secondary metabolites. Table 2 lists the secondary metabolites that were extracted from a few plants employing microwaves. Silver nanoparticle stability is provided by the comparatively high concentrations of steroids, sapogenins, carbohydrates, and flavonoids found in plant extracts, which function as reducing agents and phytoconstituents as capping agents.
Amino group-containing proteins are thought to influence synthesis and lower silver ions. It was discovered that the protein’s secondary structure had changed following the contact. The manufacture of metal nanoparticles can benefit greatly from the utilization of plants and their extracts. This is because antioxidants found in plant extract have the potential to function as reducing agents. The concentration of plant extract and AgNO3, the most popular precursor for the synthesis of AgNPs, can be changed to adjust the size and form of the nanoparticles. The biosynthesis will be more advantageous and a large quantity of nanoparticles may be produced if they are made outside of the cell. The presence of carbonyl groups in aspartic acid and/or glutamine residues, as well as the hydroxyl residues of proteins that are in charge of metallic ion reduction, is thought to be the potential reaction mechanism for the production of NPS using plants.
The production of nanoparticles from different extracts derived from medicinal plants is a simple, affordable, and biocompatible method. Because they contain secondary metabolites, medicinal and herbal plants are a natural supply of medications. Since ancient times, these have been utilized as traditional and folk medicine. Interestingly, secondary metabolites are effectively employed in the production of many kinds of nanomaterials. Terpenoids, alkaloids, flavonoids, and polyphenolic chemicals including catechin, epicatechin, flavones, taxifolin, procyanidins, and others are examples of secondary metabolites that are utilized in the nanoscale production of materials. When it comes to green synthesis of nanomaterials, flavonoids are the most often reported chemicals. Flavone, flavanone, flavanonol, and isoflavone derivatives are members of the flavonoids family of naturally occurring polyphenolic chemicals. These substances are important to plants because they help them react to both biotic and abiotic stress.
Secondary metabolites are typically used to cap the green produced nanoparticles, giving the nanomaterials more stability. In addition to their stability, some metallic nanomaterials can have their biological activity increased by the presence of secondary metabolites. Interestingly, green produced ZnO NPs have anti-diabetic properties. Even so, we have covered a number of benefits of green nanoparticle formation and secondary metabolites. It is important to note a few drawbacks of green synthesis here. When plants are exposed to harmful concentrations of nanoparticles, oxidative stress is frequently documented. The destruction of cellular macromolecules, particularly membrane lipids, which results in cell death, is a frequent effect of dangerous ROS levels. NPs have the ability to harm DNA and other macromolecules. mitochondrial malfunction and DNA damage. Cell apotosis are observed in eggplants. The recent studies indicate that NPs could affect microbial and plant secondary metabolism.
The majority of the algae belong to the kingdom Protista and are aquatic photosynthetic organisms. The life cycles of algae range widely, from tiny mass to enormous kelp. Protein is abundant in algae. As a result, scientists and researchers in the food science field frequently try to use algae as a protein supplement for people. It becomes challenging to grow enough food for everyone because of the population growth and land scarcity. For this reason, the efficient use of algae as food is being emphasized. It is important to note that some types of algae are high in protein and may even have therapeutic properties. One well-known example of algae being utilized as medicine is spirulina. Apart from, the food use these algae can be used to synthesize nano-materials. Recently, Singaravelu et al. [93] adopted a systematic approach to study the synthesis of metallic nanoparticles by an algae Sargassum wightii. This is the first report where the marine algae have been used for the synthesis of highly stable extracellular gold nanoparticles in a relatively short period of time compared with that of other biological procedure. Indeed, reports mention that 95% of bio-reduction of AuCl4- ions occurred within 12 hours at stirring condition. The same author has synthesized palladium and platinum nanoparticles using their corresponding metallic chloride salts.
The location of the reductive component of the cell affects microbial metal nanoparticle production. When cell wall reductive enzymes are engaged in the reductive process of metal ions, extracellular nanoparticles are a foregone conclusion. Extracellular nanoparticle manufacturing has more applications in optoelectronics, electronics, bioimaging, and sensor technologies than intracellular nanoparticle production. The prokaryotic bacteria Rhodopseudomonas capsulata has been discovered to degrade Ag+3 to Au0 extracellularly at room temperature. When the pH of the solution was kept constant at 7.00, spherical nanoparticles were made, and when the pH of the solution was changed, nanoparticles of various shapes and sizes were formed. The participation of one or more proteins in the bio-reduction and capping of gold nanoparticles can be shown using polyacrylamide gel electrophoresis (PAGE).
Due to its optical and semiconducting properties, selenium is utilized in photocopiers and microelectronic circuit devices. Two selenite and selenium-breathing bacteria, Sulfurospirillum barnesii and B. selenitri, produce elemental selenium (Se0), which has a monoclinic crystalline structure and is stable and evenly organized [43, 47– 50, 94, 95]. Its extracellular diameter is approximately 300 nm. As will be discussed below, the two main kinds of bacterial nanoparticle synthesis are intracellular and extracellular synthesis.
i) Intracellular synthesis of nanoparticles by bacteria Additional processing processes, including ultrasonic treatment or reaction with appropriate detergents, are necessary to separate the intracellularly produced nanoparticles. Precious metals can also be recovered using this technique from metal leachates and mining debris. Additionally, metal nanoparticles may be employed in bioreactors as a catalyst in a variety of chemical reactions. Bacterial activity has been linked to the deposition of mineral ores in recent years. There have been reports of the iron and manganese oxide deposition process using pedomicrobium, or budding bacteria. They are now utilized in Alaska because it has been discovered that they may gather gold. Bascillus subtilus 168 reduced Au3+ ions to Au0 and deposited them inside their cell walls in the dimensions of 5-25 nm producing octahedral morphology. Interestingly, sulphate reducing bacteria in gold mines was used to destabilize gold (l) – thiosulphate complex (AuS2O3)23- to produce elemental gold (<10nm) in the bacterial envelope releasing H2S as end product of metabolism.
In Fe (III) reducing bacterium known as Geobacter Ferrireducers gold was precipitated intracellularly in the periplasmic space. Similarly, in anaerobic conditions microbial reduction and deposition of gold nanoparticles was achieved at 25oC over the pH range of 2 to 7 using the mesophilic bacterial Shewanella algae in the presence of H2 as electron donor. The reductive deposition of gold by the resting cells of S. algae was a fast process and it produced nanoparticles of gold with 10-20 nm in periplasmic space (at pH=7.0) and with 15-200 nm on bacterial surface (at pH=2.8).
Similarly, silver nanoparticles with different single crystals structure such as equilateral triangular and hexagonal with particle size up to 200 nm have been produced in periplasmic space of bacterium Pseudomonas stutzeri AG259, popularly known as silver mine bacterium. This bacterium also produced a small quantity of nanoparticles with monoclinic crystalline structure from silver sulphate (Ag2S) as a base material. This approach produced crystalline nanoparticles of silver and sulphur in the ratio of 2:1. Bio-absorption and bio-reduction of Ag (1) on cell surface was also reported to occur in about 24 hours for bacterium lactobacillus sp. A09 at a temperature of 30oC and pH of about 4.5. Similarly dried cells of coryobacterium species SH09 produced silver nanoparticles at 60oC in 72 hours on the cell wall in the size range of 10-15 nm with diamine silver complex [Ag(NH3)2]+ as the base material.
Periplasmic silver binding proteins, which bind silver at the cell surface and shield the cytoplasm from toxicity, have often proved successful in reducing silver toxicity. Silver binding proteins that supply amino acid moieties, which act as a nucleation site for the creation of silver nanoparticles, are thought to be present in the organic matrix. Ag+ was recently found to be reduced to Ag0 by an airborne Bacillus sp. that was isolated from the atmosphere. There have been reports of certain bacteria producing bimetallic alloys and multiple nanoparticles. Nair and Pradeep discovered that Lactobacillus sp. in butter milk generated tiny, well-defined gold, silver, and gold-silver bimetallic nanocrystals in their cells without compromising their viability.
Numerous microbes can synthesize nanoparticles with a variety of shapes, including cubic, hexagonal, and spherical, within the size range of 5 to 200 nm. For instance, B. subtilis, bacteria that produce sulphate, S. algae, P. boryanum, E. Coli, R. Capsulatus, and others can synthesize gold and silver nanoparticles. A number of investigations on the bacterial manufacture of gold nanoparticles have been conducted by Absar Ahmad and his research team. In one study, they effectively synthesized monodispersed gold nanoparticles using extremophilic actinomycetes called Thermonospora sp. In different investigations, these scientists synthesized high-quality monodispersed nanoparticles intracellularly using alkalotolerant Rhodococcus sp [96– 101].
ii) Extracellular synthesis of nanoparticles by bacteria
The location of the reductive component of the cell determines the microbial synthesis of metal nanoparticles. It is evident that extracellular nanoparticles are produced when the reductive enzymes found in cell walls are engaged in the reductive process of metal ions. Compared to intracellularly created nanoparticles, extracellular synthesis offers a greater range of applications in optoelectronics, electronics, bio-imaging, and sensor technology. It has been discovered that the prokaryotic bacteria Rhodopseudomonas capsulata may extracellularly degrade Ag+3 to Au0 at room temperature. When the pH of the solution was kept at 7.00, this approach produced spherical nanoparticles; when the pH was changed, nanoparticles of different sizes and shapes were created. Poly-acrylamide gel electrophoresis (PAGE) can be used to reveal the involvement of one or more proteins in the bio-reduction and capping of the gold nanoparticles. Selenium has optical and semi-conducting properties and finds their applications in photocopiers and microelectronic circuit devices. There are few selenite and selanate respiring bacteria such as sulfurospirillum barnesii and B. Selenitri which synthesize stable, uniform shaped elemental selenium (Se0) with monoclinic crystalline structure [85, 102–106] of diameter of about 300 nm extracellularly.
In many respects, fungi are superior to other microbes. In contrast to plant materials and bacteria, fungal mycelia mesh can tolerate flow pressure, agitation, and other conditions in bioreactors or other chambers. Extracellular enzymes are secreted by fungi with remarkable efficiency. Therefore, it is simple to produce enzymes on a big scale. Economic viability and simplicity of processing biomass are two more benefits of employing a fungal-mediated green strategy for the synthesis of metallic nanoparticles. However, the genetic modification of these eukaryotic organisms to overexpress particular enzymes poses a serious risk to the use of these bio-entities in the creation of nanoparticles.
i) Intracellular synthesis of nanoparticles using fungus
The nanoparticles produced inside the body might be smaller than those that are reduced outside of cells. Their size is related to the size of the particles that develop inside the organism. Gold nanoparticles were created using verticillium sp. This work described the formation of approximately 20 nm-diameter gold nanoparticles in the cytoplasm membrane of a fungal mycelium. These generated nanoparticles’ size and variation are well described. It was found that the cells of another fungus, Trichothecium sp., could contain gold nanoparticles. This found that silver nanoparticles accumulated on the cell wall surface of Aspergillus flavus after treating the fungus with a silver nitrate solution for 72 hours.
ii) Extracellular synthesis of nanoparticles by fungus There are several advantages of synthesising nanoparticles outside of cells compared to inside. Since fungi have significant secretary components that can reduce and cap nanoparticles, it is usually believed that they are the organisms that produce nanoparticles extracellularly. F. acumintum, which was isolated from infected ginger, produced silver nanoparticles through extracellular mycosynthesis. These generated nanoparticles had a spherical form and ranged in size from 5 to 4 nm. Thrichoderma asperellum has been reported to create stable, well-defined silver nanoparticles in the 13–18 nm size range [47, 50–53].
A virus is a submicroscopic infectious agent that only replicates inside living cells, according to microscopic understanding. They are little bits of genetic data that are shielded by capsids. There are viruses that have envelopes. The term “naked virus” refers to a virus that has no envelope. They resemble parasites quite a bit. Compared to human cells, the virus is 100–1000 times smaller. The virus can only replicate within the cells of its living host. They can infect bacteria, fungi, plants, animals, and people. Each exclusively infects a particular kind of host. Interestingly, viruses may also infect bacteria; this is called bacteriophage. The efficacy of the virus in treating bacterial diseases that do not react to bacterial infection is being investigated by researchers. There are several ways to categorize viruses, including (1) their appearance, which includes their size and shape, such as spherical, complicated, helical, icosahedral, or polyhedral. (2) the characteristics of their genome, such as DNA or RNA. DNA functions similarly to a virus’s construction handbook. The translation of instructions into a language that the cell machinery can understand and convert into protein is what RNA is. Viral DNA may be circular or linear. (3) their structural characteristics, and (4) according to whether an envelope is there or not.
The debate over whether a virus is living or not is an intriguing one. Based on all the features, viruses appear to be inanimate objects. One of the essential traits of living things is metabolism, which is absent. Viruses, on the other hand, exhibit replication, a special characteristic of living things. As a result, researchers occasionally think of viruses as existing on the boundary between living and non-living things. We are fairly aware of the several infectious diseases that viruses can cause these days. Covid-19 in humans and lumpy skin disease in cattle are two recent pandemic diseases that have been propagated by viruses. For viral illnesses, there are frequently no effective treatments available.
As a result, physicians are always working to control viral diseases through preventive measures. The standard procedure for preventing many viral infections is vaccination. It’s worth noting that viruses can be employed to efficiently create materials at the nanoscale. Recently, a novel method of using viruses and their capsids for biotechnology has started to shift toward applications in nanotechnology. For their investigation, researchers Douglas and Young were the first to take into account the plant virus cowpea chlorotic mottle virus. Protein cages called viruses are used to produce new nanomaterials in a very regulated and exact manner. Select amino acids on virus capsids can be added or removed through genetic and chemical alteration for applications ranging from mineralization to bio-conjugation.
Scientists have been interested in creating plant viruses as bio-templates for the synthesis of nanomaterials in recent years. Applications for virus-mediated synthesized nanomaterials are numerous and include biomedicine, sensing, and more. Imaging can be done with the nanomaterials produced via virus-mediated technology. The method utilized to examine the internal structure and malfunction of bodily parts is called magnetic resonance imaging. MRI techniques can make use of the virus-mediated produced nanomaterials. In our imaging approach, paramagnetic gadolinium is used as the contrast agent. The use of plant viruses to deliver drugs is still in its infancy. Plant viruses can be effectively employed in medication delivery systems without additional reproduction.
The therapeutic payload is either coupled with the exterior or interior capsids or placed inside the inner cavity for use in nanomedicine. Numerous chemotherapeutics that have received clinical approval are delivered using virus nanoparticles. Among the many benefits of complexing therapeutic drugs with viral nanoparticles are improved pharmacokinetics and, of course, improved drug delivery to sick areas. According to the literature review, viruses are utilized in a novel way to create synthetic nanocrystals such zinc sulfide, silicon dioxide, cadmium sulfide, and ferrous oxide. The synthesis of nanocrystals using biological methods has been expanded to include whole biological particles. Inorganic nanomaterial nucleation and assembly can be modeled using viral scaffolds, according to reports.
In fact, Cowpea Chlorotic Mottle Virus (CCMV) and cowpea mosaic virus (CMV) have been used as nucleation cages for the mineralization of inorganic materials. Furthermore, tobacco mosaic virus has been shown to successfully mineralize lead sulphide (PbS) and cadmium sulphide (CdS) to synthesize crystalline nano-wires [107–109].
Reports of the synthesis of metallic nanoparticles by yeast are scarce. Candida glabrata is a eukaryotic bacterium that has mostly been used to synthesize semiconductor nanoparticles. It generated intracellularly monodispersed, spherically shaped, peptide-bound CdS quantum crystallites with a size of 20 A0. By combining with phytochelatins to generate metal-thiolate complexes, it counteracted the toxicity of cadmium metal ions. The utilization of yeast to create FCC-structured PbS quantum nanocrystallites with semiconductor characteristics was initially documented by Kowshik et al. [110], it was discovered that another yeast, S. cerevisiae, could make spherical, face-centered PbS nanoparticles. It was discovered that antimony oxide (Sb2O3) nanoparticles with a cubic structure and a room temperature size of 2–10 nm exhibited semiconductor characteristics [110, 111].
Template-mediated synthesis of inorganic nanoparticles and microstructures has made use of biological materials such as DNA, protein cages, bio-lipids, viroid capsules, bacterial rapidosomes, S-layers, and multicellular super structures. It is thought that glutamate and aspartate on the virus’s outer surface aid in the creation of nanoparticles. Genetically modified virus capsids that self-assembled were used as biological templates to manufacture quantum dot nanowires. A dual peptide virus-engineered capsid can also be used to create hybrid nanowires (ZnS-CdS) [112, 113].
Nanotechnology may find methods for producing nanoparticles that use naturally occurring reagents including vitamins, sugars, plant extracts, biodegradable polymers, and microorganisms as capping agents and reductants appealing. Plant-based materials appear to be the best options among the reagents listed above and are appropriate for the large-scale production of nanoparticles. For the manufacture of metal nanoparticles, plant components such leaves, roots, latex, seeds, and stems are utilized. Polyphenols found in tea, wine, winery waste, red grapes, pomace, and other materials are thought to be the main active ingredients in certain syntheses.
Metal ion complexation with a suitable bio-ligand in its native oxidation state or after reduction to a lower oxidation state are two ways that metals can accumulate internally in plants. Material scientists are interested in using plants to synthesize nanoparticles and control their size and form because of the potential for plants to reduce metal ions and the existence of metal complexing agents in them. In addition to being a cost-effective method that could be scaled up for large-scale synthesis, the strategy would aid in preventing the escalating concerns about biological dangers and environmental damage. Among the different methods used for metallic nanoparticles synthesis, biosynthesis using plants is of peculiar interest because they are environmentally benign as well as they require smaller synthesis time rather than elaborate process of maintaining cell culture required in micro-organism assisted synthesis.
Plant extracts are easily combined with a room-temperature metal salt solution to create nanoparticles. Within minutes, the reaction is finished. This method has been used to create gold, silver, and numerous other metal nanoparticles. It is known that the type of plant extract, its content, the metal salt concentration, pH, temperature, and contact duration all influence how quickly and how well nanoparticles are produced. A Cinnamomum comphora leaf extract can be used to create nanoparticles by reducing silver and gold ions. The extract’s flavones, polysaccharides, terpenoids, and phenolic components were credited with the reduction. According to reports, the bactericidal activity of these nanoparticles peaks at a concentration of 45.
The presence of H+ ions, NAD+, and ascorbic acid in the extract was attributed to the reduction of silver ions used to create nanoparticles utilizing extracts of Desmodium trifolium. It has been claimed that Datura leaf extract can be used to synthesize extremely stable silver nanoparticles. The reduction of silver ions to nanoparticles is thought to be caused by alkaloids, proteins, enzymes, amino acids, alcoholic chemicals, and polysaccharides found in this plant’s extract. The extract’s quinol and chlorophyll pigments also helped to stabilize the nanoparticles and reduce the amount of silver metal. Within eight minutes, silver ions were reduced to nanoparticles (4.30 nm) by an extract of Ocimum santum leaves. This high activity of the extract was ascribed to the relatively high levels of ascorbic acid contained in the extract. In another study silver nanoparticles produced using O. santum leaf extract have been found to have a high antimicrobial activity against both gram negative (E. coli) and Gram positive (Streptococcus aureus) microorganisms. S. Shiv Shankar has synthesized silver and gold nanoparticles using Azadirachta indica leaf extract. It resulted in the formation of mono disperse nanoparticles. Presence of reducing sugar was speculated to contribute to stabilizing the nanoparticles. Gold and silver nanoparticles have been produced using extract of Aloe vera and Comellina sinensis.
Capping agents and templates: Frequently the technique developed for synthesis of one metal nanoparticle could also be applied for other metals. Fabrication of metal, metal oxides and salt nanoparticles using natural substances is a very promising area in nanotechnology, which come to prominence only a few years ago. Useful materials can be produced easily even in reasonable scale because the biomaterials based routes eliminate the need to use harsh and toxic chemicals. Waste products are relatively innocuous and easier to dispose of because they are mostly composed of leaf-over natural plant extracts. The majority of research has taken place for the synthesis of silver nanoparticles; perhaps due to their easier preparation, importance in disinfection science and utility in several applications. Nanoparticles of several metals, their alloys, oxides and salts have been obtained by greener methods.
In these greener routes, products derived from plant such as extracts of various plants, tea, coffee, banana, simple amino acids, wine, table sugar and glucose have been utilized as reductants and capping agents. A significant number of efforts have been taken for the use of tea extracts, which owing to a number of poly-phenols present; as chelating or reducing and capping agents for the nanoparticle synthesis. Therefore, the resulting particles are protected from further reactions and aggregation, which increases their stability and longevity. Greener method involves plant materials that have been used in nanoparticles synthesis are generally single pot reactions without the use of additional surfactant [114– 116].
Nanoparticles can be toxic in nature even though their bulk counterparts are nontoxic. Nanotoxicology is the study of toxicity of nanomaterials. The toxicity can arise in two ways; first nanoparticles can trigger on adverse immune response system of human body, and second acting as a poison. The immune system is a system of biological structure and process within an organism that protects against disease. To function properly the immune system must detect a wide variety of agents known as pathogens like viruses and parasites and distinguish them from organism’s own healthy tissue. The immune response triggering by an artificial material (nanoparticle) in contact with blood and tissue typically arises because proteins dissolved in blood and other body fluids adsorb onto the surface of material and change their conformation. The native protein is thereby transformed into a foreign protein, recognized as such by circulating immune cells, which trigger the usual apparatus for eliminating foreign invaders into action. Any immovable artificial material will become a permanent site of inflammation.
Usually, poisoning has a particular biological process. Usually, a toxin attaches itself to the enzyme’s active site and stops it from binding its usual substrate. The classic example is carbon monoxide, which outcompetes oxygen binding by binding hemoglobin’s heme group. In their elemental state, most chemicals are not poisonous. A bacterium called mercury reductase is employed to simply reduce mercury in order to prevent the treat from consuming mercury ions.
These two possible sources of toxicity will be made worse by materials in nano form in three ways. First, a block of material’s surface area is greatly increased if it is split up and distributed as nanoparticles. As a result, an immunological trigger that may have been minor turns into a serious one. Second, compared to bulk metal, nanoparticles ionize more easily. The atoms of lead and silver that separate and ionize from the substrate block are what cause the toxicity, not the metal in its elemental form. Thirdly, because of their small size, nanoparticles can pass through materials that would be impossible for larger particles to do so. The two main types of barriers seen in the human body are a densely packed layer of cells and a lipid bi-layer that surrounds individual cells. Here is evidence that nanoparticles can pass between cells arranged in such tightly packed layers such as those containing blood brain barrier, and that they can pass through the lipid bi-layer into the cytoplasm of individual cells much as some macromolecules of comparable size are able to do [117, 118].
The propensity of germs to cause community-acquired diseases has made antibiotic resistance a global concern. Numerous strategies have been proposed to address the issue of antibiotic resistance. In addition to using antibiotics appropriately for both therapeutic and non-therapeutic purposes, research on safe and efficient antibacterial drugs has also been promoted. Therefore, several inorganic nanoparticles, including copper, zinc oxide, titanium, silver, and gold, have been investigated for their potential application as antibacterial agents.
Actually, silver and its compounds have long been known for their antibacterial properties, and they have been used as antibiotics to treat wounds, burns, and various infectious disorders. Because of their enormous surface areas and distinct physical, chemical, and biological characteristics, silver nanoparticles exhibit superior antibacterial properties compared to the bulk metal. As a result, silver nanoparticles are more efficient against a variety of bacteria, fungi, and viruses than silver compounds such as silver nitrate, silver sulphadiazine, etc. Silver nanoparticles based on substrates like mesoporous silica, zeolite etc. are capable of slow and controlled release of silver ions which render them more effective for long term antibacterial activity. Thus, silver nanoparticles possess wide range of applications as an antibacterial agent in hygiene, cosmetic and medical applications and antibacterial water filtering.
Chlorination has been a key strategy to stop bacterial development in provided water worldwide since it was first used to disinfect drinking water at the turn of the 20th century. But throughout the water treatment process, chlorine, being a potent oxidant, interacts with organic matter to create a number of byproducts. Human cancer risk may rise as a result of these byproducts. Furthermore, bacteria’ resistance to traditional chemical disinfectants necessitates a very high disinfectant dosage, which exacerbates the generation of disinfectant byproducts. But the potable water used in developing countries particularly in rural areas are generally collected from untreated sources that are far away from home and stored for long time before use usually have lesser disinfectant ability at the time of use. Thus, innovative disinfection methods to enhance the reliability and efficiency of disinfection while avoiding disinfectant by-products are required.
Silver nanoparticles have been used as an antibacterial water filter in a number of investigations. This employs ceramic filter membranes for antifouling and silver nanoparticles coated on polyurethane foams that are anchored on methacrylic acid as co-polymer beads and have granular activated carbon incorporated. Therefore, materials containing silver nanoparticles are a good substitute for treating water, especially at the point of consumption [119, 120].
Human eyes cannot see nanoparticles due to their minuscule size. The human eye has a resolving power of 1’ and a magnification capacity of up to 10-3M. Therefore, it is impossible to directly view nanoparticles. The activity of nanoparticles is determined by their size and form. Therefore, sophisticated instrumental approaches can be used to study it indirectly. The two sorts of instruments utilized for analysis are 1) spectroscopes and 2) microscopes. The scientific field of spectroscopy studies how electromagnetic radiation interacts with matter. The most effective method for examining atomic and molecular structure is spectroscopy, which is employed to analyze a variety of materials. Electromagnetic radiations are widely used in for spectroscopic technique of characterization and those are listed in Table 3.
| Electromagnetic Radiation Wave Length | Application | Orientation | Frequency | Wavelength |
| Gamma rays | Ionization and Gamma garden | Nuclear | 1021 | 3×10-11 |
| X rays | XRD | Removal of inner shell electrons | 1019 | 3×10-9 |
| Ultraviolet rays | UV-visible spectroscopy | Removal of outer shell electrons | 1017 | 3×10-7 |
| Visible | UV-visible spectroscopy | Excitation of valence electrons | 1015 | 3×10-5 |
| Infra-Red | IR/ Raman absorption spectroscopy | Molecular vibration | 1013 | 3×10-3 |
| Microwaves | EPR | Magnetically induced spin state | 1011 | 3×10-1 |
| Radio | NMR | Spin rotation | 109 | 3×101 |
A versatile method utilized in many fields of research, technology, pharmacy, and veterinary medicine is UV-visible spectrophotometry is also used to gain insight into the rate at which nanoparticles form, the chemical characterization of noble metals, and the production of nanomaterials. A sample’s strength of absorption of visible and near-ultraviolet radiation can be measured using UV-visible spectroscopy, as seen in Figure 13. It calculates the quantity of distinct UV-visible light wavelengths that a sample absorbs or transmits when compared to a reference or blank sample. The sample makeup affects this characteristic, which may yield both qualitative and quantitative data. UV-visible radiation ranges in wavelength from 200 to 800 nm and is energetic enough to promote outer electrons in an atom to higher energy levels [97]. Moreover, UV-visible spectroscopy can also be used to determine the band gap of semiconductors.
As UV radiations are associated with high energy, it produces a change in the electronic energy of the molecule. Since spectroscopic techniques relies on light. The energy of electromagnetic waves is directly proportional to its frequency and inversely proportional to its wavelength. All electromagnetic waves travel with same speed if medium is same.
In the meantime, the energy difference between the ground state and the excited state determines how much energy is absorbed; the smaller the difference, the less energy is needed to promote the electron from the ground state to the excited state, and eventually, the longer the absorption wavelength. The wavelength of radiation with the energy needed for an electronic transition is represented by the location of an absorption band.
Metallic nanomaterials typically have distinct metallic lusters and distinctive colors. The coherent oscillation of valence band electrons brought on by interaction with an electromagnetic field is the source of metallic nanoparticles’ absorption of electromagnetic radiation. These resonances, which are exclusive to nanomaterials, are referred to as surface plasmons. UV-visible spectroscopy is a useful technique for characterizing the Surface Plasmon Resonance phenomena that is present in noble metal colloidal solutions [100].

The energy difference between the ground state and excited state determines how much energy is absorbed; the lower the difference, the longer the absorption wavelength. An absorption band’s position and intensity are its primary characteristics. The wavelength of radiation with the energy needed for an electronic transition is represented by the location of an absorption band. The likelihood of interaction between the radiation energy and the electronic system, as well as the difference between the ground state and excited state, are the two main elements that determine the degree of absorption. Beer-Lambert’s law can be used to determine the intensity of absorption, which is expressed as
Beer Lambert’s law: “When a bean of monochromatic light is passed through homogeneous absorbing medium then the rate of decrease of intensity of radiation with the thickness of absorbing medium is directly proportional to the intensity of radiation as well as concentration of solution.”
The metallic nanomaterials are known to exhibit characteristic colour. The absorption of electromagnetic radiation by metallic nanoparticles originates from coherent oscillation of valence band electrons induced by an interaction with electromagnetic field. These resonances are known as surface plasmon that occurs only with nanomaterials. The noble metal colloidal solutions are showing the phenomenon of Surface Plasmon Resonance, thus UV-visible spectroscopy is a powerful tool for its characterization.
UV-Visible spectroscopy is an important technique for chemical analysis of noble metallic nanomaterials. In this research work, this technique has been used to get an idea about the formation of nanomaterials. Also this technique can be used to reveal rate of formation of nanoparticles [121].
Wilhelm Conrad Rontgen, a German physicist, made the discovery of X-rays in 1895. Since their nature was unclear at the time, they were referred to as X-rays. X-rays are electromagnetic radiations with a wavelength significantly shorter than visible light that are invisible and highly penetrating. Electromagnetic radiations with wavelengths of 10-10 meters are known as X-rays. In 1912, Van Lave proved that crystals could diffract X-rays. Le Galley went on to build the first X-ray powder diffractometer in 1935. Since its creation, metallurgists and mineralogists have mostly used X-ray powder diffractometers to examine structural flaws. X-Rays used in material characterization is shown below in Figure 14 and Figure 15.
Scientists and researchers studying materials must ascertain their crystalline structure and chemical makeup. The chemical composition, crystal structure, crystal orientation, crystallite size, lattice strain, phase composition, preferred orientation of powder, solid, and liquid samples, and layer thickness can all be determined using x-ray diffraction, a non-destructive and precise laboratory technique that is currently available. Several materials are made up of tiny crystallites. The chemical composition and structure of these tiny crystallites are called phase. The materials can be single phase or multiple phase mixture. The materials can contain crystallite and non-crystalline compositions at the same time. distinct crystallite shapes produce distinct x-ray patterns when the sample is exposed to x-ray diffraction patterns. The diffraction pattern can be compared to identify the phase. ICDD now maintains the most extensive database. Wilhelm Conrad Rontgen72, a German scientist, made the discovery of X-rays in 1895. Since their nature was unclear at the time, they were referred to as X-rays. X-rays are electromagnetic radiations with wavelengths far shorter than visible light that are invisible and extremely penetrating. Electromagnetic radiation with a wavelength of 10-10 m is what X-rays are. In 1912, Van Lave proved that crystals could diffract X-rays. Le Galley later built the first X-ray powder diffractometer73 in 1935.
Since its creation, X-ray powder diffractometers have been used mostly by mineralogists and metallurgists to examine structural flaws. When X-rays interact with the electrons in the materials they are bombarded with, they might scatter or glow. This phenomenon can provide details about an object’s elements as well as its detailed structure. Metallurgy and material sciences have made extensive use of X-ray diffraction studies.
The quick analytical method known as X-ray powder diffraction is mostly employed to determine the correct phase and crystalline material. After finely grinding and homogenizing the sample under analysis, the average bulk composition is ascertained. Usually, high-energy electron bombardment of a metal produces them. In order to interact with the inner electron shell, the high-energy electrons must pass through the outer shells. An inner-shell electron is ejected, or escapes the nucleus’s attractive field, if more energy is provided to it than is necessary. This leaves the hole in the inner shell and creates an ionized atom. The ionized atom can return almost to its lowest energy (ground state) by filling in the missing electron with one from the outer shells. It is this transmission that is accompanied by the emission of an X-ray or Auger electron. It is the transition which is accompanied by the emission of an X-Ray or Auger electron.


Fourier transform spectroscopy is a method for measuring the spectrum of a substance by using light interference as opposed to light dispersion. This spectroscopic method is often used to determine the different functional groups found in organic molecules. The use of FT-IR can be expanded to material science, where it can be effectively used to identify the reduction process of metal ions. The identification of organic molecules is greatly aided by spectroscopy in the middle infrared range.
Because of the intricate structure that yields a large number of maxima and minima, this method is frequently employed in organic chemistry. For spectral detection and analysis, contemporary spectrometers operating in the infrared spectrum often employ the Fourier transform technique. A molecule’s atoms vibrate around their mean positions rather of staying in a single relative position. This vibrational motion makes such modes of vibration IR active if the dipole moment alternates periodically. The wavelength range of the infrared portion of the electromagnetic spectrum is 100µm to 1µm. Only radiations of their oscillation frequency, or those with which they can interact coherently, cause the vibrating molecules to absorb energy.
As a result, every functional group has unique vibrational frequencies that are highly responsive to its surrounding species and chemical environment. The presence or absence of specific vibrational frequencies can be used to infer a compound’s molecular structure. Vibrational frequency is the to-and-fro motion of a particle about its mean position. The idea behind vibrational spectroscopy, also known as infrared spectroscopy, is that molecules absorb particular light frequencies that correspond to their structural makeup. The mass of its atoms, the vibronic coupling, and the molecular surface’s shape all affect the energies.
The key concept in FTIR is the use of a mathematical operation called Fourier transformation which converts a signal in the time domain to the frequency domain. The frequency-domain transformation speeds up the estimation of the IR spectrum of the signal. The time required here is 1 sec or less vs 10-15 minutes for the normal system. The reduction in time allows for taking several sample scans and thereby improving the resolution and quality of the FTIR imaging. In FTIR spectroscopy, we are using the term wave-number which is reciprocal to wavelength.
The FTIR image may also be impacted by the capping agent selection. The vibration modes of the capping molecules will alter as they are adsorbed on the surface of the nanoparticle. As a result, the functional group’s vibrational frequency characteristics will either shift, and the degree of this shifting will depend on the type and intensity of interactions.
FTIR Instrumentation: Infrared spectrometer has similar components to those of ultraviolet and visible instruments. The essential components of an infrared spectrometer are
In FT-IR, a Globar serves as the light source. Silicon carbide in the form of rods or aches of various diameters is the material of choice for Globar. A broadband emitter, such as a mid-IR ceramic source, a mid- and near-IR tungsten-halogen source lamp, or a far-IR mercury lamp that offers good intensity across a wide range, is usually employed as the light source for an FTIR spectrometer.
Reflection optics is used in FT-IR spectroscopy to view and concentrate infrared light onto the sample. The degree of transparency and refractive index are crucial factors in choosing a material for optical applications. Calcium fluoride, fused silica, germanium, magnesium fluoride, potassium bromide, sapphire, sodium chloride, zinc selenide, zinc sulfide, and so on are the most often employed optical materials in FT-IR.
There are different types of sample holders for FT-IR spectroscopy. Herein we will discuss various types of sample holders in short:
(a) Universal holder: This is a versatile all round shape holder for transmission analysis. This type of sample holder can be used for films, pellets, windows, mulls etc.
(b) K-holder: Another kind of sample holder is this one. You can use this sample holder for pellets, liquid samples, and more. To properly position the sample based on its unique size and shape, the base and positioning mount can be moved up and down. All spectrophotometer kinds can use the rear plate, which is of standard size.
(c) EZ-Clip: This is basic and easy to use sample holder to effortlessly hold windows, pellets and films in place. The spring loaded clips hold samples firmly at proper place. The standard size back plates fit in all spectrophotometers.
(d) Quick holder: Collars for 1, 3, 7, and 13mm KBr pellets can be held in the sample holder. The sample is held in place by the magnetic back plate. 13mm pellets fit through a slot at the top of the holder.
The detectors can be classified in three different categories such as thermal detector, pyroelectric detectors and photo-conducting detectors. Finally, the response of the detector is measured using some indicating instrument.
For spectral detection and analysis, they typically employ the Fourier transform approach while working in the infrared spectrum. Michelson’s interferometer, which is the fundamental component of an FTIR instrument, is made up of a stationary and a moveable mirror, as seen in Figure 16. Albert A. Michelson built Michelson’s interferometer in 1887. The scientific community came to believe that all waves require a medium in order to propagate as a result of Michelson’s interferometer experiments. The subject of how light waves reach Earth from the Sun was then brought up. It was assumed that the hypothetical medium between the earth and the sun existed in order to respond to this question. This hypothetical medium is termed as luminiferous aether or ether medium. Principle of working of Michelson’s interferometer: A Michelson’s interferometer works by using a half silvered mirror to split an incoming wave into two equal waves. The waves after splitting are sent in different perpendicular directions. These two waves after travelling a particular distance, encounter a plane mirror and is sent back to the silver mirror, where the two light waves are then directed to an observation screen or detector and the two light waves half recombine and produce an interference pattern. This interference pattern and how it changes during an experiment, can be analyzed to make the measurements in different fields.
Usually, a Nernst filament made up of a spindle of rare-earth oxides serves as the source. After that, the beam is directed and forced through a beam splitter, which is a component of the interferometer that transmits the remaining radiation while reflecting roughly half of it. Depending on the path difference produced by the movable mirror, the beam that has been split into two pieces will either recombine constructively or destructively. Therefore, an actual signal is made up of radiation that spans a wide range of wave numbers, and the sum of the individual contributions determines the overall strength.

Nuclear spin (I), which can be integral (1, 2, 3, …) or odd half integral (1/2, 3/2, 5/2…), is present in all atomic nuclei. The spinning nucleus creates a magnetic field whose axis coincides with the spin axis because the nucleus has a positive electric charge. Therefore, each nucleus can be thought of as a tiny magnet with a magnetic moment \(\mu\) that is proportional to spin. Every nucleus with an I value greater than zero has a magnetic moment. Only a specific set of orientations can be assumed by a magnetic dipole when a magnetic nucleus is exposed to a homogeneous magnetic field. The system is quantized in this case. A magnetic nucleus can have any of the following (2I + 1) orientations with respect to the applied magnetic field direction.
NMR spectroscopy requires strong magnetic fields. Tesla (T) is the international unit of magnetic flux. Although it fluctuates, the earth’s magnetic field is roughly 10-4T at ground level. Powerful magnets with fields ranging from 1 to 20T are used in modern NMR spectrometers. Nuclei in the +1/2 state will be excited to the higher -1/2 spin state when a sample is exposed to radio frequency (RF) energy that precisely matches the spin state separation of a particular set of nuclei. The broadcast spectrum for radio and television includes this electromagnetic energy. Therefore, the energetically mildest probe for analyzing a molecule’s structure is NMR spectroscopy.
The absorption peak in NMR signal is dependent on external magnetic field strength as well as electromagnetic wave frequency irradiated. Since no two magnets have exactly the same magnetic field, resonance frequencies will vary accordingly and alternative method for characterizing and specifying the location of NMR signal is needed [122, 123].
The technical discipline of using a microscope to see things and substances that are invisible to the naked eye is known as microscopy. Electron, optical, and scanning probe microscopy are the three well-known subfields of microscopy. In optical and electron microscopy, electromagnetic radiation or an electron beam interacts with the object through diffraction, reflection, and refraction. The dispersed radiation or another signal is then collected to produce a picture. Wide field irradiation of the sample (such as TEM) or scanning a narrow beam across the sample (such as SEM) are two methods that can be used to accomplish this operation.
The resolving power of a light microscope, or its capacity to discern two neighboring spots as separate and distinct, is its primary restriction. It is not helpful to merely magnify an object without being able to discern structural elements. A microscope’s maximum magnification might not be the most helpful because the resulting image could be hazy or blurry. Therefore, a high-resolution photograph is preferred for improved clarity. The wavelength of light employed and the lens system’s numerical aperture (NA) determine a microscope’s resolving power. Numerical Aperture: The numerical aperture of a microscope is a measure of its ability to gather light and resolve fine specimen detail at a fixed object distance.
An optical device is the animal eye. It converts photons into electrical signals that people can understand and identify as color and light. Engaging in visual perception without the use of a magnifying glass or other light-collecting optical device, like a telescope or microscope, is known as “naked eye.” When observing a close item without a magnifying glass or microscope, the object’s size is determined by the viewing distance. The angular size that can be seen with the naked eye in typical light or brightness conditions is approximately 1 arc minutes = 1/60 degrees = 0.003 radians. Some external assistance is necessary if one want to visualize objects smaller than this. Microscope is the instrument used for magnification and resolution of the smaller objects. Microscopy is the science of investing small objects and their structure using such an instruments. Microscopy means invisible to eye unless aided by the instrument microscope. A significant contribution in the area of microscope is devoted by Dutch Physicist Antonie Philips van Leeuwenhoek. Later on much advancement has taken place in the area of microscopes.
The technical discipline of using a microscope to view materials and objects that are invisible to the naked eye is known as microscopy. Scanning probe, optical, and electron microscopy are the three well-known subfields of microscopy. In optical and electron microscopy, electromagnetic radiation or an electron beam interacts with the object through diffraction, reflection, and refraction. The dispersed radiation or another signal is then collected to produce a picture. A narrow beam can be scanned across the sample (as in a scanning electron microscope) or the sample can be exposed to wide-field radiation (as in a transmission electron microscope). Table 4 provides the following summary of the microscopy’s developmental scenario:
| 167 BCE | The Chinese use water made of a lens and water-filled tube to visualize the unseen. |
| 1000 AD | Reading stone. The first vision aid called the reading stone was invented. It is a glass sphere placed on the top of the text which magnifies readability. |
| 1021 | Optics book: Muslim scholar Ibn al Hytham writes his book on optics. |
| 1284 | The first eyeglass was invented. Salvino Darmate invented the first wearable glass |
| 14th century | Spectacles were first made in Italy |
| 1590 | Dutch spectacle maker Hans Jansen and his son Zacharias Jansen were claimed by later writers to have invented the compound microscope |
| 1609 | Galileo Galilei developed a compound microscope with a convex and a concave lens |
| 1619 | Cornelius Drebbel presents, in London a compound microscope with two convex lens |
| 1620 | Christian Huygens, another Dutchman developed a simple 2 lens ocular system that was chromatically corrected. |
| 1665 | Robert Hooke published Micrographia a collection of biological micrographs. He coins the word cell for the structures he discovers in cork bark |
| 1674 | Anton van Leeuwenhoek improved a simple microscope for visualizing biological objects. He was the first person to visualize bacteria. |
| 1830 | Joseph Jackson Lister discovered that using weak lenses together at various distances provided clear magnification. |
| 1878 | Ernest Abbe developed a mathematical theory linking resolution to light wavelength. |
| 1903 | Richard Zsimondy invented the ultramicroscope, which allows for observation of specimens below the wavelength of light. |
| 1931 | Ernest Ruska started to build the first electron microscope. It is a Transmission Electron Microscope. |
| 1932 | Transparent biological materials were studied for the first time using Frits Xernike’s invention of the phase-contrast microscope. |
| 1938 | James Hillier built another TEM |
| 1953 | Zernaik, a professor of theoretical Physics was awarded the Nobel prize for the invention of the phase-contrast microscope |
| 1955 | George Nomarski, professor of microscopy published the basis of differential interference contrast microscopy |
| 1981 | Gerd Binning, Qutar, and Gerber invented Atomic Force Microscopy |
| 1988 | Kingo Itaya invented the Electrochemical Scanning Microscope |
| 1991 | Kelvin, probe the force microscope invented |
a) Resolving power: The ability of an optical device to distinguish between two adjutant lines or points is known as its resolving power. The minimal distance between two lines or points that can still be distinguished decreases as the resolving power increases. A light microscope’s resolving power is its primary drawback. It is not helpful to merely magnify objects without being able to discern structural features. A microscope’s maximum magnification might not be the most helpful because the resulting image could be hazy or blurry. Therefore, a high-resolution photograph is preferred for improved clarity. The refractive index, the lens system’s numerical aperture (NA), and the wavelength of light employed all affect a microscope’s resolving power.
b) Objective Numerical Aperture: The numerical aperture of a microscope is a measure of its ability to gather light and resolve fine specimen detail at a fixed object distance.
Compared to a typical microscope, a scanning electron microscope offers numerous advantages. Because of its wide depth of field, SEM can focus on a larger portion of the specimen at once. SEM is displayed in Figure 17. This kind of electron microscope uses a concentrated electron beam to scan a sample and create photographs of it. The sample’s atoms and electrons interact to produce a variety of detectable signals that reveal details about the composition and surface topography of the sample. An image is created by combining the position of the electron beam with the signal it detects while scanning in a raster scan pattern. Closely spaced specimen samples can be magnified at much higher levels thanks to the SEM’s significantly higher resolution.
Because scanning electron microscopy has a greater depth of focus and resolution than optical microscopes, it is very helpful for direct surface studies. At a far higher magnification than a light microscope can provide, the SEM displays incredibly detailed 3-D images. A black-and-white image is produced when light waves are absent. To put it succinctly, SEM operates in a vacuum inside a column emitting a high-energy electron beam from an electron gun. The electron column and control console are the two main parts of a SEM. Electrons moving down an evacuated tube are influenced by the electron column’s two or more electron lenses and electron cannon. Scanning Electron Microscopy is extremely useful for direct observation of surface because they offer better resolution and depth of field than optical microscope. The two major component of SEM are electron column and control console. The electron column consists of an electron gun and two or more electron lenses, which influences the path of electron travelling down an evacuated tube.
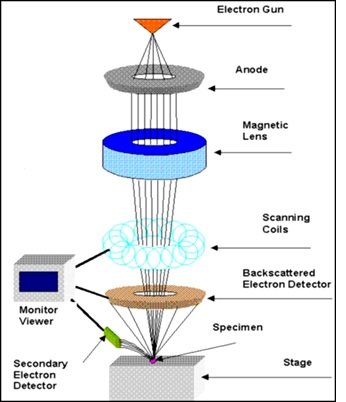
Instrumentation: A SEM consists of an electron gun that produces the electrons, an anode at high voltage that accelerates the electrons, a system of electromagnetic lenses that focuses the beam of electrons onto the samples, scanning coils that facilitate the scanning of electron beam over the sample surface, the sample chamber where the sample is located, and detectors that measures the signals generated due to the interaction of the electrons with sample and this is shown in Figure 18. All these components are housed within the vacuum chamber.

These are two types of electron source or electron gun; the first thermionic and the second field emission guns. A thermionic electron relies on electrons emitted from heated wire or a filament. The filament is usually a bent tungsten wire that functions as a cathode. The bent portion is heated up when an electric current passes through the filament. The outer orbital electrons of the tungsten atoms are emitted when they gain sufficient thermal energy to overcome the energy barrier that prohibits the electrons from escaping. The higher the temperature more is the number of electrons emitted. Tungsten is usually chosen as filament because it can withstand high temperature without melting. However, thermionic electron guns have relatively low brightness. Whereas the field emission electron gun relies on electrons emitted from a sharp tip upon the application of a high electric field. It does not involve heating of a filament. Instead, when a high electric field is applied to the tip, quantum mechanical tunneling takes place. Typically, the field emission gun has two anodes. The first anode (at 0-5 KV) serves to extract the electrons from the tip while second anode (at 1-50KV) serves to accelerate the electrons and this determines the energy of electrons travelling down the column of SEM. The field emission gun has higher brightness.
As electrons are streaming out of electron guns, they form a spray pattern. In order to control the profile of this electron beam into a finely adjusted focused beam, electromagnetic lenses are used. When an electron with charge q and velocity v travels in a region with a magnetic field B it experiences a force.
Here it should note that since the direction of force acting is perpendicular to the direction of the velocity, therefore the Lorentz force acting on the electrons has no effect on the speed of electrons. The only effect the magnetic field has is on the electron which changes the direction of motion of electrons.
The magnetic profile generated by a typical electromagnet used in SEM is highly non-uniform. The magnetic field of electromagnetic lens can be considered to be made up of two independent components viz. the vertical axis component (Hz) and horizontal radial component (Hr). The radial component causes the electron travelling in 2-direction to move in a helical manner with respect to the central axis. The axial component causes the electron to move closer to the central axis, i.e. the effect of axial component is to reduce the diameter of the helical path of the electrons. As a result, the electron beam spirals down the column as it passes through the electromagnetic lens. The resultant effect is that the electron beam becomes finely focused and can be scanned over the sample for imaging purposes.
The scanning of the electron beam over surface of sample is achieved by deflecting the beam using an applied electrostatic or magnetic field. Typically, a deflection yoke consists of four radially oriented coils arranged so that the magnetic field is perpendicular to the axis of the system. The magnetic field generated by these coils can be controlled by the amount of electric current passing through these coils. By programming these coils, one can readily focus the electron beam over the sample surface.
The typical accelerating voltage used in SEM is of the order of a few thousand volts. With an energetic beam of electrons scanning over the sample surface, a number of phenomenon occur due to interactions between the electrons and sample atoms. For example; the incident electron can collide with the electron of the atoms or atomic nuclei. Energetic incident electrons can collide with the electrons of sample and knock them out of their usual orbits. These electrons are known as secondary electrons. During the process, the incident electron loses little energy and continues to generate more secondary electrons as it travels further into the sample. A single incident electron will generate a shower of thousands of secondary electrons until the secondary electron loses its energy. Since large number of secondary electrons is generated, the detection of secondary electron is the most common mode of operation for SEM sample imaging. Secondary electrons are having low energy so that secondary electrons generated deep in the sample are unable to travel to the surface and leave the sample. As a result, the secondary electrons detected are primarily from region close to the sample surface (< 10 nm). Thus SEM imaging would produce good topographical information for the sample.
Sometimes, an incident electron collides with the nucleus of a sample atom, causing the electron to bounce back. Such electrons are referred to as backscattered electrons. Since the atomic nucleus is more massive than electron, the back scattered electrons has high velocity and are characterized by their high energy of a few KeV. High density samples will generally create more back scattered electrons, and hence back scattered electrons can be utilized to identify difference in the densities of the sample. The production of back scattered electrons is directly proportional to the atomic number of the atoms in the sample. Therefore, the regions with atoms of higher atomic number would appear brighter than regions with atoms of lower atomic number. Thus besides providing the information of topography back scattered electrons provides valuable information on the density and elemental distribution in the sample [124].
Working Principle: TEM is a microscopic technique in which a beam of electron is transmitted through an ultra-thin specimen, interacting with the specimen as it passing through. An image is formed from the interaction of the electrons transmitted through the specimen; the image is magnified and focused onto the imaging device such as florescent screen. In TEM analysis, a thin specimen is illuminated with electrons in which the electron intensity is uniform over the illuminated area. As the electrons travel through the specimen, they are either scattered by a variety of processes or they may remain unaffected by the specimen. The end result is that a non-uniform distribution of electrons emerging from the surface of the specimen contains all the structural and chemical information about the specimen. Electron microscope is constructed to display this non-uniform distribution of electrons in two different ways viz. angular distribution of scattering that can be viewed in the form of scattering patterns, and spatial distribution of scattering that can be observed as contrast in images of the specimen. The advantages of this arrangement are the possibility of directly viewing the area from which the diffraction pattern arises. The basic components of the transmission electron microscope are shown in Figure 19.

A technical explanation of a typical TEM working is as follows:
1. The vital source i.e. an electron gun produces a stream of monochromatic electrons.
2. The stream is focused to a small, thin coherent beam by the use of condenser lenses 1 and 2. The first lens is usually controlled by a spot size knob that largely determines the spot size and the second lens is usually controlled by the intensity or brightness knob that actually changes the size of the spot on the sample.
3. The beam is restricted by the condenser aperture, knocking out high angle electrons.
4. The beam strikes the specimen and part of it is transmitted.
5. The transmitted portion is focused by the objective lens.
6. Optical objective and selected area metal apertures can restrict the beam, the objective aperture enhancing contrast by blocking out high-angle diffracted electrons, the selected area aperture enabling the user to examine the periodic diffraction of electrons by ordered arrangement of atoms in the sample.
7. The image is passed down the column through the intermediate and projector lenses.
The image strikes the phosphorus image screen and light is generated, allowing the user to see the image. The darker area of the image represents those areas of the sample that fewer electrons were transmitted through [125].
Particle size distribution is also known as grain size distribution. Particle size of nanomaterials influences many properties. It is a valuable indicator of their quality and possible applications. This concept hold valid for all phases of materials such as powder, suspension, emulsion and aerosol. If the particle size is small, then its solubility increases which leads to higher surface charge (zeta potential). The particle size analysis not only reveals the average particle size but also other statistical distribution parameters such as mode, median, distribution width etc. which are equally important. Particle size measurement is routinely carried out across a wide range of industries and is often a critical parameter in the manufacture of many products. Particle size has a direct influence on material properties such as reactivity, stability, texture, viscosity, and porosity. Particle size analysis is the statistical distribution of the particles of different sizes. It is common practice to represent this distribution in the form of either a frequency distribution curve or a cumulative distribution curve. Particle size distribution can be represented in many ways such as number-weight, volume-weight and intensity-weight distribution.
Number-weight distribution: A counting technique such as image analysis will give a number-weight distribution where each particle is given equal weighting irrespective of its size. This is most important where knowing the absolute number of particle is important.
Volume-weight distribution: Static light scattering technique such as laser diffraction will give a volume-weight distribution. Here the contribution of each particle in the distribution relates to the volume of that particle; i.e. relative contribution will be proportional to the (size)3. This is very useful from a commercial perspective.
Intensity-weight distribution: dynamic light scattering technique will give an intensity-weight distribution where the contribution of each particle in the distribution relates to the intensity of light scattered by particle; for example, using the Rayleigh approximation the relative contribution for very small particles will be proportional to the (size)6.
When comparing particle size data for the same sample measured by different techniques it is important to realize that the type of distribution being measured can produce very different particle size results. This can be illustrated in the example as shown in figure 1.20.
The novel properties of NPs have been exploited in a wide range of potential applications in medicine, cosmetics, renewable energies, environmental remediation and biomedical devices. Among them silver and copper nanoparticles have attracted increasing interest due to their unique physical, chemical and biological properties compared to their macro-scaled counterparts. These metallic NPs have distinct physico-chemical properties including a high electrical and thermal conductivity, surface enhanced Raman scattering, chemical stability, catalytic activity and non-linear optical behavior. These properties make them of potential value in consumer products like ink, microelectronics and medical imaging. A primary interest in the idea of nanoscience comes from its associations with biology. The size of the nanoparticle is comparable to the size of the biomolecules such as DNA, enzymes, antibodies, and polysaccharides. Noble metal nanoparticles like gold and silver are found to be bio-compatible. Therefore, they find various medicinal applications.
Miller, D. (2007). Stone age or plastic age?. Archaeological Dialogues, 14(1), 23-27.
Prasad, R. D., Sarvalkar, P. D., Prasad, N., Prasad, R. S., Prasad, R. B., Prasad, R. R., … & Guo, Z. (2024). Emerging trends of bioactive nano-materials in modern veterinary science and animal husbandry. ES Food & Agroforestry, 18, 1144.
Love, J. C., Estroff, L. A., Kriebel, J. K., Nuzzo, R. G., & Whitesides, G. M. (2005). Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chemical Reviews, 105(4), 1103-1170.
Prasad, R. D., Sahoo, A. K., Shrivastav, O. P., Charmode, N., Prasad, S. R., Kamat, R., … & Prasad, N. R. (2022). A review on aspects of nanotechnology in food science and animal nutrition. ES Food & Agroforestry, 8, 12-46.
Jones, R. M. (2018). Mechanics of Composite Materials. CRC press.
Prasad, R. D., Charmode, N., Shrivastav, O. P., Prasad, S. R., Moghe, A., Sarvalkar, P. D., & Prasad, N. R. (2021). A review on concept of nanotechnology in veterinary medicine. ES Food & Agroforestry, 4(5), 28-60.
Feynman, R. P. (1960). There’s plenty of room at the bottom. Engineering and Science, 23(5), 22-36.
Prasad, R. D., Desai, C. B., Srivastava, O. P., Prasad, S. R., Bhat, T. S., Kamble, B., … & Prasad, R. Y. (2023). A critical review on recent developments in advanced supercapacitors for veterinary medicine. ES Food & Agroforestry, 11, 805.
Ravi, B. (2003). Investment Casting Development.
Prasad, R. D., Prasad, N. R., Prasad, R. S., Prasad, N., Prasad, S. R., Shrivastav, M., … & Kamble, J. (2024). A review on nanotechnology from prehistoric to modern age. ES General, 4, 1117.
Ravi, B. (2003, September). Investment casting development: ancient and modern approaches. In National Conference on Investment Casting Central Mechanical Engineering Research Institute, Durgapur.
Prasad, R. D., Prasad, S. R., Shrivastav, O. P., Kanthe, A. R., Waghmare, S. R., Shaikh, V. S., … & Shaikh, Y. I. (2023). Biogenic synthesis of nano-silver and their anti-microbial efficacy. ES Food & Agroforestry, 11(2), 836.
Day, L. (2006). Carbon Nanotechnology: Recent Developments in Chemistry, Physics. Materials Science and Device Applications. Amsterdam, Oxford, Elsevier.
Prasad, R. D., Prasad, R. S., Shaikh, Y. I., Prasad, S. R., Padvi, M. N., Sarvalkar, P. D., … & Patil, P. D. (2024). A critical review on uses of gases in veterinary medicine and gas sensing materials. ES Materials & Manufacturing, 23, 1084.
Roco, M. C. (2001). From vision to the implementation of the US National Nanotechnology Initiative. Journal of Nanoparticle Research, 3(1), 5-11.
Prasad, R. D., Prasad, R. S., Prasad, R. B., Prasad, S. R., Singha, S. B., Singha, D., … & Navathe, G. J. (2024). A review on modern characterization techniques for analysis of nanomaterials and biomaterials. ES Energy & Environment, 23, 1087.
Maye, M. M., Lou, Y., & Zhong, C. J. (2000). Core-shell gold nanoparticle assembly as novel electrocatalyst of CO oxidation. Langmuir, 16(19), 7520-7523.
Prasad, R. D., Teli, B., Prasad, R. S., Prasad, R. B., Prasad, S. R., Sinha, P., … & Guo, Z. (2024). A review on thin film technology and nanomaterial characterization techniques. ES Materials & Manufacturing, 25, 1198.
Friend, R., Burroughes, J., & Shimoda, T. (1999). Polymer diodes. Physics World, 12(6), 35-40.
Prasad, R. D., Prasad, N. R., Prasad, R. B., Prasad, R. Y., Prasad, S. R., Gour, M., … & Sarvalkar, P. (2023). A critical review on bio-mimetic synthesis of transition metal nanoparticles: their biomedical applications in veterinary medicine. ES Food & Agroforestry, 15, 1027.
Carpi, F., De Rossi, D., Kornbluh, R., Pelrine, R. E., & Sommer-Larsen, P. (Eds.). (2011). Dielectric Elastomers as Electromechanical Transducers: Fundamentals, Materials, Devices, Models and Applications of an Emerging Electroactive Polymer Technology. Elsevier.
Prasad, R. D., Prasad, R. J., Shrivastav, R. K., Charmode, N., RS, P., Mamidpelliwar, P. M., … & Sarvalkar, P. (2023). A review on concept of veterinary bio-technology and livestock products in medicine. ES Food & Agroforestry, 14, 1004.
Ozin, G. A., Arsenault, A. C., & Cademartiri, L. (2009). Nanochemistry: A Chemical Approach to Nanomaterials. Royal Society of Chemistry, London.
Prasad, R. D., Desai, C. B., Shrivastav, O. P., Charmode, N., Prasad, S. R., Samant, A., … & Sarvalkar, P. (2022). A critical review on design and development of carbonaceous materials for veterinary medicine. ES Food & Agroforestry, 9, 15-38.
Fesmire, S. (2003). John Dewey and Moral Imagination: Pragmatism in Ethics. Indiana University Press, Bloomington, Indiana.
Prasad, R. D., Sonawane, K. D., Prasad, R. S., Prasad, S. R., Prasad, N., Shrivastav, R. K., … & Guo, Z. (2024). A review on livestock viral disease and their management. ES General, 5, 1227.
Chaudhuri, R. G. (2009). Synthesis and Characterization of SiAgBr Core Shell Nanoparticles (Doctoral dissertation, M. Sc. Thesis).
Prasad, R. D., Prasad, N. R., Prasad, R. S., Prasad, R. R., Prasad, B., Prasad, S. R., Prasad, N., … & Patil, M. B. (2024). A critical review on electron, X-ray, probe microscopic techniques for analysis of biomaterials and nanomaterials. International Journal of Nanomaterials and Nanostructures, 10(2), 1–32.
Kapoor, S. S. (2005). Hinduism. Hemkunt Press, New Delhi, 110028, India.
Prasad, R. D., Prasad, N. R., Prasad, R. S., Shrivastav, O. P., Prasad, S. R., Banga, S., Desai, C. B., … & Prasad, R. R. (2024). A review on nanotechnological aspects in medicinal textile. Advances in Analytic Science, 5(1), 2694.
Hahnemann, S. (2002). Organon of Medicine. B. Jain publishers, Uttar Pradesh 201309, India.
Prasad, R., Prasad, N., Prasad, N., Prasad, R., Prasad, S., Shrivastav, O., Prasad, R., Prasad, R., Prasad, R., & Saxena, P. (2024). A review on phenomenon of adsorption of inorganic materials: Applications in veterinary pharmacology and material sciences. Advances in Analytic Science, 5(2), 3000.
Galarraga Soto, E., & Luna Hermosa, G. (1984). Criterios de diseño para servicios básicos mínimos de agua potable en barrios suburbanos. Revista Técnica Informativa. XIX aniversario IEOS, 26-30.
Freestone, I., Meeks, N., Sax, M., & Higgitt, C. (2007). The Lycurgus cup—a roman nanotechnology. Gold Bulletin, 40(4), 270-277.
Link, S., Wang, Z. L., & El-Sayed, M. A. (1999). Alloy formation of gold-silver nanoparticles and the dependence of the plasmon absorption on their composition. The Journal of Physical Chemistry B, 103(18), 3529-3533.
Steinke, J. M., & Shepherd, A. P. (1988). Comparison of Mie theory and the light scattering of red blood cells. Applied Optics, 27(19), 4027-4033.
Singh, H., & Pal Singh, B. (2013). “The next big thing is the really small. Nanotechnology: A conceptual study”. International Journal of Information Technology & Computer Sciences Perspectives, 2(3), 606-612.
Kelly, K. L., Coronado, E., Zhao, L. L., & Schatz, G. C. (2003). The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. The Journal of Physical Chemistry B, 107(3), 668-677.
Eckert, J., Das, J., Pauly, S., & Duhamel, C. (2007). Mechanical properties of bulk metallic glasses and composites. Journal of Materials Research, 22(2), 285-301.
F Dorfman, B. (2010). Some Trends and Challenges in Nanomechanics: Up-To-Date Review of Selected Patents and Patent Applications. Recent Patents on Mechanical Engineering, 3(3), 191-205.
Lanas, J., & Alvarez-Galindo, J. I. (2003). Masonry repair lime-based mortars: factors affecting the mechanical behavior. Cement and concrete research, 33(11), 1867-1876.
Ovid’ko, I. A. (2005). Superplasticity and ductility of superstrong nanomaterials. Reviews on Advanced Materials Science, 10(2), 89.
Lokwani, P. (2011). Magnetic particles for drug delivery: an overview. International Journal Of Pharmaceutical And Bio-Medical Science, 2(2), 465-473.
Barnes, W. L., Dereux, A., & Ebbesen, T. W. (2003). Surface plasmon subwavelength optics. Nature, 424(6950), 824-830.
Yushanov, S. P., Gritter, L. T., Crompton, J. S., & Koppenhoefer, K. C. (2012). Surface Plasmon Resonance. In COMSOL Conference, Amsterdam.
Shipway, A. N., Katz, E., & Willner, I. (2000). Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. ChemPhysChem, 1(1), 18-52.
Mulvaney, P. (1996). Surface plasmon spectroscopy of nanosized metal particles. Langmuir, 12(3), 788-800.
Chang, Y. A. (1967). On the temperature dependence of the bulk modulus and the Anderson-Grüneisen parameter \(\delta\) of oxide compounds. Journal of Physics and Chemistry of Solids, 28(4), 697-701.
Yguerabide, J., & Yguerabide, E. E. (1998). Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications: I. Theory. Analytical biochemistry, 262(2), 137-156.
Link, S., & El-Sayed, M. A. (1999). Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. The Journal of Physical Chemistry B, 103(21), 4212-4217.
Burda, C., Chen, X., Narayanan, R., & El-Sayed, M. A. (2005). Chemistry and properties of nanocrystals of different shapes. Chemical Reviews, 105(4), 1025-1102.
Halas, N. J., Lal, S., Chang, W. S., Link, S., & Nordlander, P. (2011). Plasmons in strongly coupled metallic nanostructures. Chemical reviews, 111(6), 3913-3961.
Bansmann, J., Baker, S. H., Binns, C., Blackman, J. A., Bucher, J. P., Dorantes-Dávila, J., … & Xie, Y. (2005). Magnetic and structural properties of isolated and assembled clusters. Surface Science Reports, 56(6), 189-275.
Gupta, A., & Sun, J. Z. (1999). Spin-polarized transport and magnetoresistance in magnetic oxides. Journal of Magnetism and Magnetic Materials, 200(1), 24-43.
Tarling, D., & Hrouda, F. (Eds.). (1993). Magnetic Anisotropy of Rocks. Springer.
Ju-Nam, Y., & Lead, J. R. (2008). Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Science of the Total Environment, 400(1), 396-414.
Mahmoudi, M., Hofmann, H., Rothen-Rutishauser, B., & Petri-Fink, A. (2011). Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chemical Reviews, 112(4), 2323-2338.
Pankhurst, Q. A., Connolly, J., Jones, S. K., & Dobson, J. (2003). Applications of magnetic nanoparticles in biomedicine. Journal of physics D: Applied physics, 36(13), R167.
Prasad, R. D., Sarvalkar, P. D., Prasad, N., Prasad, S. R., Prasad, R. S., Prasad, R. B., … & Ghosh18, J. (2024). A Review on Spectroscopic Techniques for Analysis of Nanomaterials and Biomaterials. ES Energy & Environment, 27, 1264.
Prum, R. O., Quinn, T., & Torres, R. H. (2006). Anatomically diverse butterfly scales all produce structural colours by coherent scattering. Journal of Experimental Biology, 209(4), 748-765.
Narayanan, R., & El-Sayed, M. A. (2003). Effect of catalysis on the stability of metallic nanoparticles: Suzuki reaction catalyzed by PVP-palladium nanoparticles. Journal of the American Chemical Society, 125(27), 8340-8347.
Burda, C., Chen, X., Narayanan, R., & El-Sayed, M. A. (2005). Chemistry and properties of nanocrystals of different shapes. Chemical Reviews, 105(4), 1025-1102.
Cheong, S., Watt, J. D., & Tilley, R. D. (2010). Shape control of platinum and palladium nanoparticles for catalysis. Nanoscale, 2(10), 2045-2053.
Motta, C. (2013). First-Principles Study of Electronic Transport in Organic Molecular Junctions (Doctoral dissertation, Università degli Studi di Milano-Bicocca).
Ferry, D. K., & Goodnick, S. M. (1997). Transport in Nanostructures (No. 6). Cambridge University Press.
Ramos, A., Morgan, H., Green, N. G., & Castellanos, A. (1998). Ac electrokinetics: a review of forces in microelectrode structures. Journal of Physics D: Applied Physics, 31(18), 2338.
Bethe, H. A. (1949). Theory of the effective range in nuclear scattering. Physical Review, 76(1), 38.
Cushing, B. L., Kolesnichenko, V. L., & O’Connor, C. J. (2004). Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chemical Reviews, 104(9), 3893-3946.
Tao, A. R., Habas, S., & Yang, P. (2008). Shape control of colloidal metal nanocrystals. Small, 4(3), 310-325.
Kent, S. B. (1988). Chemical synthesis of peptides and proteins. Annual Review of Biochemistry, 57(1), 957-989.
Zangwill, A. (1988). Physics at Surfaces. Cambridge University Press.
Akiyoshi, K., Kobayashi, S., Shichibe, S., Mix, D., Baudys, M., Wan Kim, S., & Sunamoto, J. (1998). Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: complexation and stabilization of insulin. Journal of Controlled Release, 54(3), 313-320.
Hogg, R. T. W. D. W., Healy, T. W., & Fuerstenau, D. W. (1966). Mutual coagulation of colloidal dispersions. Transactions of the Faraday Society, 62, 1638-1651.
Cordes, E. H., & Dunlap, R. B. (1969). Kinetics of organic reactions in micellar systems. Accounts of Chemical Research, 2(11), 329-337.
Green, M. (2010). The nature of quantum dot capping ligands. Journal of Materials Chemistry, 20(28), 5797-5809.
Toshima, N., & Yonezawa, T. (1998). Bimetallic nanoparticles—novel materials for chemical and physical applications. New Journal of Chemistry, 22(11), 1179-1201.
Wang, W., Efrima, S., & Regev, O. (1999). Directing silver nanoparticles into colloid-surfactant lyotropic lamellar systems. The Journal of Physical Chemistry B, 103(27), 5613-5621.
Parak, W. J., Gerion, D., Pellegrino, T., Zanchet, D., Micheel, C., Williams, S. C., … & Alivisatos, A. P. (2003). Biological applications of colloidal nanocrystals. Nanotechnology, 14(7), R15.
Dahl, J. A., Maddux, B. L., & Hutchison, J. E. (2007). Toward greener nanosynthesis. Chemical Reviews, 107(6), 2228-2269.
Katz, E., & Willner, I. (2004). Integrated nanoparticle–biomolecule hybrid systems: synthesis, properties, and applications. Angewandte Chemie International Edition, 43(45), 6042-6108.
West, J. L., & Halas, N. J. (2000). Applications of nanotechnology to biotechnology: Commentary. Current opinion in Biotechnology, 11(2), 215-217.
Slowing, I. I., Trewyn, B. G., Giri, S., & Lin, V. Y. (2007). Mesoporous silica nanoparticles for drug delivery and biosensing applications. Advanced Functional Materials, 17(8), 1225-1236.
Lu, A. H., Salabas, E. E., & Schüth, F. (2007). Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angewandte Chemie International Edition, 46(8), 1222-1244.
Lavan, D. A., McGuire, T., & Langer, R. (2003). Small-scale systems for in vivo drug delivery. Nature Biotechnology, 21(10), 1184-1191.
Thakkar, K. N., Mhatre, S. S., & Parikh, R. Y. (2010). Biological synthesis of metallic nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine, 6(2), 257-262.
Kelly, K. L., Coronado, E., Zhao, L. L., & Schatz, G. C. (2003). The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. The Journal of Physical Chemistry B, 107(3), 668-677.
Son, S. U., Jang, Y., Yoon, K. Y., Kang, E., & Hyeon, T. (2004). Facile synthesis of various phosphine-stabilized monodisperse palladium nanoparticles through the understanding of coordination chemistry of the nanoparticles. Nano Letters, 4(6), 1147-1151.
Judeinstein, P., & Sanchez, C. (1996). Hybrid organic–inorganic materials: a land of multidisciplinarity. Journal of Materials Chemistry, 6(4), 511-525.
Giersig, M., Pastoriza-Santos, I., & Liz-Marzán, L. M. (2004). Evidence of an aggregative mechanism during the formation of silver nanowires in N, N-dimethylformamide. Journal of Materials Chemistry, 14(4), 607-610.
Zheng, N., & Stucky, G. D. (2006). A general synthetic strategy for oxide-supported metal nanoparticle catalysts. Journal of the American Chemical Society, 128(44), 14278-14280.
Haxell, J. P., Williams, K. G., & Wilson, D. E. (1993). U.S. Patent No. 5,221,335. Washington, DC: U.S. Patent and Trademark Office.
Gilley, R. M., Meyers, W. E., Shannon, W. M., & Tice, T. R. (1986). U.S. Patent No. 4,585,482. Washington, DC: U.S. Patent and Trademark Office.
Singaravelu, G., Arockiamary, J. S., Kumar, V. G., & Govindaraju, K. (2007). A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids and Surfaces B: Biointerfaces, 57(1), 97-101.
Luo, X., Qiu, T., Lu, W., & Ni, Z. (2013). Plasmons in graphene: Recent progress and applications. Materials Science and Engineering: R: Reports,74(11), 351-376.
Kawabata, A., & Kubo, R. (1966). Electronic properties of fine metallic particles. II. Plasma resonance absorption. Journal of the Physical Society of Japan,21(9), 1765-1772.
Ahmad, A., Senapati, S., Khan, M. I., Kumar, R., Ramani, R., Srinivas, V., & Sastry, M. (2003). Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology, 14(7), 824.
Mandal, D., Bolander, M. E., Mukhopadhyay, D., Sarkar, G., & Mukherjee, P. (2006). The use of microorganisms for the formation of metal nanoparticles and their application. Applied Microbiology and Biotechnology, 69(5), 485-492.
Narayanan, K. B., & Sakthivel, N. (2010). Biological synthesis of metal nanoparticles by microbes. Advances in Colloid and Interface Science, 156(1), 1-13.
Fayaz, A. M., Balaji, K., Girilal, M., Yadav, R., Kalaichelvan, P. T., & Venketesan, R. (2010). Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomedicine: Nanotechnology, Biology and Medicine, 6(1), 103-109.
Mukherjee, P., Senapati, S., Mandal, D., Ahmad, A., Khan, M. I., Kumar, R., & Sastry, M. (2002). Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. ChemBioChem, 3(5), 461-463.
Gericke, M., & Pinches, A. (2006). Biological synthesis of metal nanoparticles. Hydrometallurgy, 83(1), 132-140.
Basavaraja, S., Balaji, S. D., Lagashetty, A., Rajasab, A. H., & Venkataraman, A. (2008). Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Materials Research Bulletin, 43(5), 1164-1170.
Nanda, A., & Saravanan, M. (2009). Biosynthesis of silver nanoparticles from< i> Staphylococcus aureus</i> and its antimicrobial activity against MRSA and MRSE. Nanomedicine: Nanotechnology, Biology and Medicine, 5(4), 452-456.
Shahverdi, A. R., Minaeian, S., Shahverdi, H. R., Jamalifar, H., & Nohi, A. A. (2007). Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria : A novel biological approach. Process Biochemistry, 42(5), 919-923.
Shahverdi, A. R., Fakhimi, A., Shahverdi, H. R., & Minaian, S. (2007). Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine: Nanotechnology, Biology and Medicine, 3(2), 168-171.
Mandal, D., Bolander, M. E., Mukhopadhyay, D., Sarkar, G., & Mukherjee, P. (2006). The use of microorganisms for the formation of metal nanoparticles and their application. Applied Microbiology and Biotechnology, 69(5), 485-492.
Dujardin, E., Peet, C., Stubbs, G., Culver, J. N., & Mann, S. (2003). Organization of metallic nanoparticles using tobacco mosaic virus templates. Nano Letters, 3(3), 413-417.
Elechiguerra, J. L., Burt, J. L., Morones, J. R., Camacho-Bragado, A., Gao, X., Lara, H. H., & Yacaman, M. J. (2005). Interaction of silver nanoparticles with HIV-1. Journal of Nanobiotechnology, 3(6), 1-10.
Nam, K. T., Kim, D. W., Yoo, P. J., Chiang, C. Y., Meethong, N., Hammond, P. T., … & Belcher, A. M. (2006). Virus-enabled synthesis and assembly of nanowires for lithium ion battery electrodes. Science, 312(5775), 885-888.
Kowshik, M., Ashtaputre, S., Kharrazi, S., Vogel, W., Urban, J., Kulkarni, S. K., & Paknikar, K. M. (2003). Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology, 14(1), 95.
Ankamwar, B., Damle, C., Ahmad, A., & Sastry, M. (2005). Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. Journal of Nanoscience and Nanotechnology, 5(10), 1665-1671.
Kim, K. S., Demberelnyamba, D., & Lee, H. (2004). Size-selective synthesis of gold and platinum nanoparticles using novel thiol-functionalized ionic liquids. Langmuir, 20(3), 556-560.
Kramer, R. M., Li, C., Carter, D. C., Stone, M. O., & Naik, R. R. (2004). Engineered protein cages for nanomaterial synthesis. Journal of the American Chemical Society, 126(41), 13282-13286.
Sharma, N. C., Sahi, S. V., Nath, S., Parsons, J. G., Gardea-Torresde, J. L., & Pal, T. (2007). Synthesis of plant-mediated gold nanoparticles and catalytic role of biomatrix-embedded nanomaterials. Environmental science & technology, 41(14), 5137-5142.
MubarakAli, D., Thajuddin, N., Jeganathan, K., & Gunasekaran, M. (2011). Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids and Surfaces B: Biointerfaces, 85(2), 360-365.
Gardea-Torresdey, J. L., Gomez, E., Peralta-Videa, J. R., Parsons, J. G., Troiani, H., & Jose-Yacaman, M. (2003). Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir, 19(4), 1357-1361.
Chung, T. H., Wu, S. H., Yao, M., Lu, C. W., Lin, Y. S., Hung, Y., … & Huang, D. M. (2007). The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials, 28(19), 2959-2966.
Brunner, T. J., Wick, P., Manser, P., Spohn, P., Grass, R. N., Limbach, L. K., … & Stark, W. J. (2006). In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environmental Science & Technology, 40(14), 4374-4381.
Nathan, C. F., & Hibbs Jr, J. B. (1991). Role of nitric oxide synthesis in macrophage antimicrobial activity. Current Opinion in Immunology, 3(1), 65-70.
Nathan, C. F., Murray, H. W., Wiebe, M. E., & Rubin, B. Y. (1983). Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. The Journal of Experimental Medicine, 158(3), 670-689.
Giusti, M. M., & Wrolstad, R. E. (2001). Characterization and measurement of anthocyanins by UV‐visible spectroscopy. Current protocols in Food Analytical Chemistry, (1), F1-2.
Sturhahn, W. (2004). Nuclear resonant spectroscopy. Journal of Physics: Condensed Matter, 16(5), S497.
Harris, R. K. (1986). Nuclear Magnetic Resonance Spectroscopy: A Physicochemical View. Harlow, Essex, England: Longman scientific & technical.
Goldstein, J., Newbury, D. E., Joy, D. C., Lyman, C. E., Echlin, P., Lifshin, E., … & Michael, J. R. (2003). Scanning Electron Microscopy and X-ray Microanalysis. Springer.
Williams, D. B., & Carter, C. B. (1996). The Transmission Electron Microscope (pp. 3-17). Springer US.
