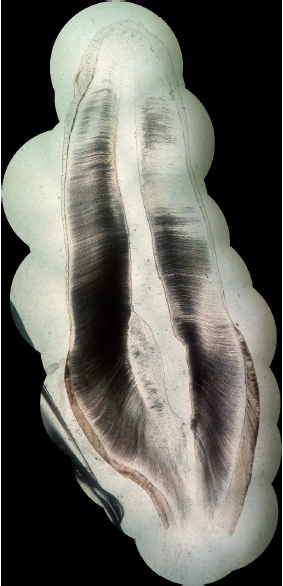
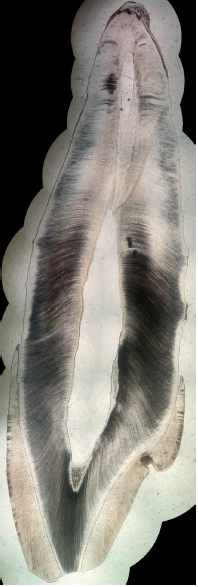
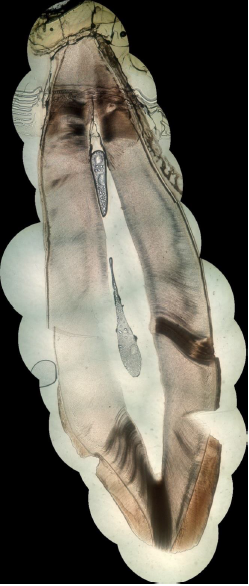
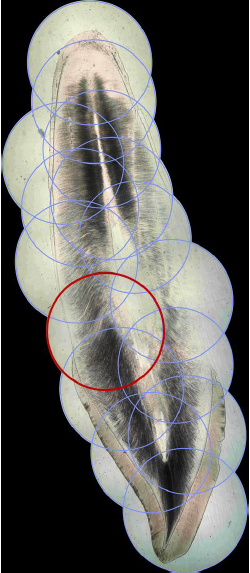
By associating last progresses in photography, computer science and additive manufacturing, cost-effective planar stitching of non-structured photographs of microscope slides into high definition large pictures is achievable. The proposed method, inspired by previous works and state-of-the art equipment, uses non-professional camera, little pre-processing, no post processing, and little to no investment is needed. A total duration of 41 min was observed to create a high-quality, high-resolution full picture of a sagittal cross-section of a permanent maxillary central incisor, from 16 original photographs with a \(\times\)40 microscope optical magnification. Final pictures weights are in-between 60 Mo and 340 Mo, depending on the format and the number of initial photographs. Higher magnification does not seem to enhance pictures, but sensibly increases file weight. This method has numerous applications, such as research, sharing and teaching and will certainly be enhanced in the future thanks to the high speed development of smartphone abilities.
Numerous areas of medicine necessitate observation and evaluation of relatively large zones of interest and with high optical magnification. These observations are generally performed with microscopes that enable to observe a very small part of the area of interest at a time.
Within the last 150 centuries, photography showed a huge development leading to very high quality pictures with relatively simple and light equipment. On the other hand, rise of digital within the last 30 years has considerably changed ways of living and working. Above all, smartphones became more than entertainment objects: they can be used as a reliable and relatively cheap professional tool in a large set of areas [1].
With the development of informatics rose the interest to digitize, annotate, share and enhance pictures obtained with glass slides on light microscopes [2]. In order to facilitate teaching class but also research work with small budget (microscope without camera), smartphone started to be investigated. Morrison and Gardner [3] proposed a simple method to take pictures with a light microscope and a smartphone, without any adapter. Despite the low cost and the easy way of implementation of this method, an operator needs some practice before managing to take clear pictures, and both hands are busy during the process. Several other authors therefore proposed hand-made and cheap adapters [4, 5]. Using an adapter to avoid blurry pictures is essential, since the outlets of this method are numerous. As an example, some authors already proposed smartphone applications in order to perform medical diagnostics [6]. Long-lasting, affordable, efficient and multi-purpose adapters are therefore needed.
As a consequence, companies started to commercialize products that are dedicated to one or several cell phones. Roy et al. [7] performed a comparison between 6 of them (and testing 3 with different smartphones), a home-made one and the direct use of the smartphone, enabling them to provide their own recommendation. On the other hand, some authors started to propose a direct printing of the adapter thanks to additive manufacturing with plastics [8]. This method has the advantage of providing the right tool with a cost-effective manufacturing process, since 3D-printers are becoming more and more affordable and worldwide.
New technologies of light microscope associated with special software tools allow for picture stitching and therefore enable creation of high-resolution pictures of the whole areas of interest, called virtual slides [9]. Thanks to multiple microscope focuses, it is even possible to obtain a 3D view of a slide (whole slide imaging) [10], which can be very useful for particular medicine areas (cytology, hematology, microbiology) [11]. However, these techniques necessitate a substantial investment for the optical tool and the software. These investments could be prohibitive for small budget institution or teaching purpose.
The objective of this work is therefore to find a way to perform 2D planar stitching of non-structured (meaning no particular organization) photographs taken by non-professional camera, with little pre-processing, no post-processing work, and little to no investment needed, in a fast way, to achieve a virtual slide with high-definition. Similar work has already been performed by Lu et al. [12] that proposed a home-made microscope and self-developed software to perform the stitching. Their method is relatively cheap (less than \(400\) and automatic however it necessitates an initial assembly step that is time-consuming and it is more adapted to remote areas that need a diagnostic system than for student classes with the need of dozens of systems.
The methodology that is presented answers that question by using a simple and cheap light microscope, a relatively recent smartphone, an adapter between microscope and smartphone that has been printed on purpose and a free image-stitching software.
The used light microscope is a simple model from Zeiss (Primo Star). It has a fixed and 20 unique objective (\(\times 10\)) and four different eyepieces (\(\times\)4, \(\times\)10, \(\times\)40 and \(\times\)10.), leading to four possible magnifications: “\(\times\)40”, “\(\times\)100”, “\(\times\)400” and “\(\times\)1000”.
The smartphone adapter has been created with a 3D printer (printer brand: Dagoma, printermodel: Neva, material: polylactic acid (PLA), material brand: ICE). The original model can be found on the website thingiverse.com [13]. It has been modified to fit with the used smartphone (Brand: Huawei, model: P20 Pro) thanks to the free platform tinkercad.com [14]. Experimental setup: smartphone, support and light microscope is shown in Figure 1.


| Action | Duration (min) |
|---|---|
| Taking pictures of the glass slides | 120 |
| Copy/Paste and conversion to .tif | 30 |
| Erasing black background | 100 |
| Stitching | 100 |
| Jpeg compression | 10 |
| Upload | 15 |
| Total | 375 |
Weights of the obtained files depend on the number of pictures that were taken in order to obtain the stitched figures. In the present case, the latter have between 20 Mpx and 250 Mpx, for a total weight of 2-10 Mo (compressed jpeg), 10-60 Mo (uncompressed jpeg) or 90-340 Mo (tif).
Given the actual glass slides, reducing the quality of the jpeg files that is given by the software to 50% allowed a 75% loss of weight with a satisfactory resolution. Choice of the compression factor is a trade-off between quality and weight. It also depends on the initial quality of the pictures and their application (for instance, cytology requires more quality, thus preventing the use of an important compression factor).
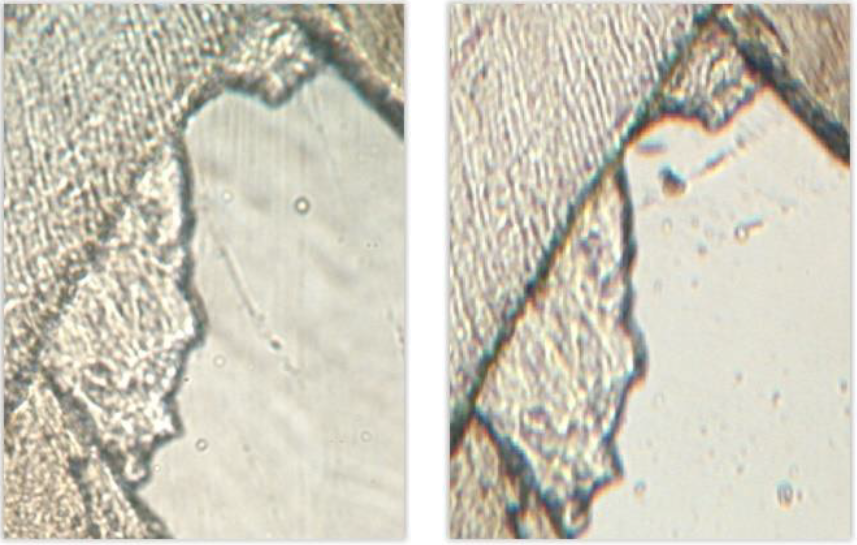
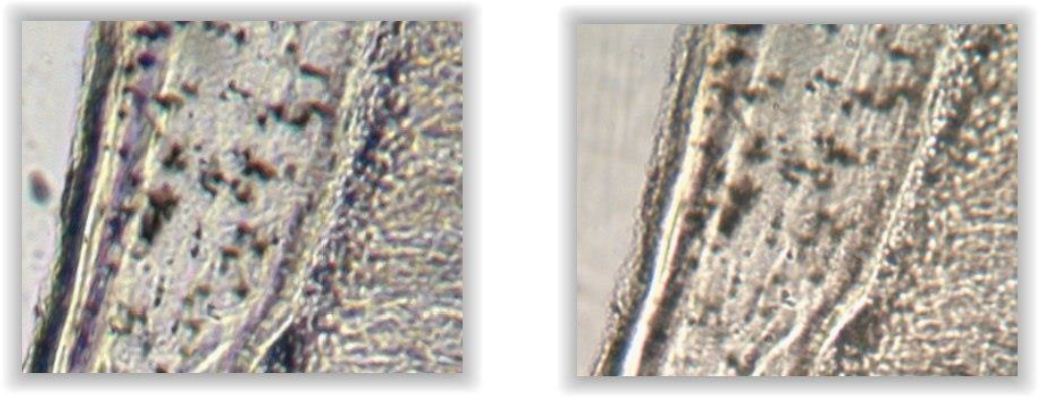
The higher magnification did not show significant enhancement of the obtained pictures, for a relatively important increase of weight (150 Mo for an uncompressed .jpeg format and 750 Mo for a .tif format, against 60 Mo and 340 Mo, respectively). However, this result should not be generalized since the potential image resolution is inherent in the optical system [16].
Even higher magnifications (\(\times\)400 and \(\times\)1000) were available with the microscope; however, the lack of multiple focus planes reduces the depth of sharpness, making such magnifications unusable for virtual slides without z-axis.

| Failure | Solution |
|---|---|
| Not enough overlap between pictures | Take more pictures (higher overlap) |
| Monotonous subject (not enough forms andcolour variations within the studied slide) |
Choosing better slides |
| Lowering light to increase variations | |
| Adding a non-monotonous element on thepicture by displacing the microscope focus |
|
| Not enough contrast | Take new pictures with better adjustment |
| Modify obtained image in post processing |
This work is in line with a global investigation about enhancement of microscope sample pictures with high quality, easy way of use and low-cost. Thanks to the progress in informatics, photography and additive manufacturing, it is feasible to meet all these requirements and create satisfying high-resolution stitched pictures of microscope glass slides with limited resources.
While previous works focused on the way of performing pictures of the microscope viewpoint with smartphone, the present method goes beyond and create large pictures that stitch them into a single high-resolution image representing the objects of interest (in the present case, teeth). Up to now, image stitching of microscope pictures was reserved to high-quality high cost microscopes and/or proprietary software products.
As a conclusion, the present works gathered all the separate knowledges that were presented to provide a simple and efficient method that can be used by researchers, teachers or students to create virtual slides that can be studied as a whole and also feed online database.
| Software | Reason |
|---|---|
| Photoshop (with tracing paper fusion) | Stitching fails: resulting image is out of shape |
| DeepSkyTracker | Necessitates numbered images and following a logical path (i.e. forbidding the use of a non-assisted by microscope protocol) |
| Fiji (ImageJ) with “stitching” plug-in | Necessitates numbered images and following a logical path (i.e. forbidding the use of a non-assisted by microscope protocol) |
| PhotoStitcher (free version) | Limited functions on free version |
| Histostitcher | Does not handle numerous images and with high overlapping |
| Image stitching | Stitching fails: resulting image is out of shape |
| Autostitch | Stitching fails: resulting image is out of shape |
| MIST | Necessitates numbered images and following a logical path |