The Taguchi Orthogonal Method was used in the study to improve biodiesel production from Jatropha oil in a single pot. This method predicted the conversion (%) from Jatropha oil transesterification by optimizing four critical process variables. Using the hydrothermal-sulphonation method, a special bio-functionalized catalyst made from agricultural waste, such as cocoa pods, eggshells, orange peels, and snail shells, was used to accelerate the reaction. The ideal conditions of MTOR (15:1), CW (3 wt%), RTime (60 minutes), and RT (65 ◦C) resulted in an optimal conversion of 95.20%. Furthermore, at MTOR of 15:1, CW of 2 Wt.%, RTime of 120 minutes, and RT of 60\(\mathrm{{}^\circ}\)C, a 99.08% product yield was obtained. Nine (9) experimental runs that assessed the FAME yield and the FFA conversion showed coefficients of variation (1.2000 and 0.1083), R\({}^{2}\) values (0.9821 and 0.9981), adjusted R\({}^{2}\) values (0.9641 and 0.9923), and projected R\(^{2}\) values (0.9091 and 0.9539), respectively. The goal of this research was to increase biodiesel yield from Jatropha oil by improving the attribute and conversion of the yielding transformation. The renewable fuel generated under peak conditions met the necessary conditions for manufacturing.
Sustainable and renewable energy sources are badly needed as a result of global population expansion, greater industrialisation, urbanisation, and economic advancement, all of which are pushing up demand for traditional biofuels. This has prompted significant study into viable replacement biofuels [1]. Renewable fuel has gained scholarly concentration as naturally viable option for petroleum-based diesel fuels [2]. This has boosted the need for clean biodiesel, which decreases environmental impact while maintaining a technically and economically viable alternative to petroleum-based diesel [3, 4]. Biodiesel is composed of mono-alkyl esters derived from long-chain fatty acids found in natural lipids like plant and animal fats. According to [5], biodiesel is created from a range of edible and non-edible feedstocks and is seen as a possible substitute for traditional diesel since it is biorenewable, non-toxic, decomposable, and has the capacity to decrease global worming [3, 6]. Biodiesel has generated global interest due to its extensive use in the transportation, industrial, and domestic sectors [7]. This issue is closely tied to feedstock costs, which can account for up to 75% of the expenditures associated with biodiesel production [8]. Reducing production costs is critical to ensuring biodiesel’s long-term viability in meeting global energy needs. Choosing non-edible oil feedstocks, such as rubber seed, 9 jatropha curcas oil, yellow oleander oil, and kapok oil, among others, is an economical approach to accomplish this [9– 12].
Studies show that good edible oil feedstocks have lower moisture content and free fatty acid (FFA) levels than poor non-edible oils [13]. Jatropha oil is a preferred second-generation feedstock among the many options available due to its high oil yield, ability to grow on limited soils, and non-edible properties. As a result, it is a desirable choice for large-scale biodiesel production without requiring land currently used for food crops [14, 15]. Nonetheless, there are downsides to using non-edible feedstocks, such as lower yields and higher manufacturing costs due to the necessity for high alcohol content [16]. To solve these constraints with non-edible oils, scientists are working hard to develop breakthrough procedures that will increase biodiesel production and open up new avenues for widespread commercial viability [17]. Although esterification and transesterification processes can be used to produce biodiesel from jatropha oil, esterification is required as the initial step when FFA levels exceed 1% [18]. In addition, biodiesel can be produced using a range of processes, including thermochemical and biochemical operations [19– 21]. Thermochemical biomass conversion uses a variety of methods, including gasification, liquefaction, transesterification, pyrolysis, and combustion. Biochemical processes include anaerobic digestion and fermentation [22– 26].
The transesterification method is an effective approach to make biodiesel. Triglycerides, which are found in a variety of oils, interact chemically with methanol or ethanol under the right conditions to form glycerol (a byproduct) and methyl or ethyl esters of fatty acids. The completed product must be thoroughly separated and purified before it is kept and utilised [27– 30].
Transesterification, separation, and purification are some of the stages required, which can be costly and time-consuming. The one-pot biodiesel technique refines the production transformation by reacting multiple reaction steps in one reactor. This optimised technique offers numerous advantages, including faster processing times, lower operating costs, and a simpler reactor architecture.
Research has shown that catalysts are critical in the transesterification process, which converts triglycerides (oils) into biodiesel. Alcohol is also required for this transformation since it serves as both a reactant and a solvent, allowing esters to form during methanolysis. The alcohol-to-triglyceride ratio might vary depending on the catalyst, oil, and process quality used. Methanol is commonly used in this procedure due to its low cost and favourable chemical and physical properties [31]
Transesterification, the process used to generate biodiesel, aims to reduce the oil’s viscosity so that it more nearly resembles regular diesel fuels. High viscosity can have a negative impact on engine performance and fuel atomisation, therefore decrease is critical. However, because transesterification is a slow process, it usually requires certain catalysts to speed up the reaction [32].
Because of their high catalytic activity, homogeneous catalysts such as NaOH, KOH, and CH\(_{3}\)ONa are commonly utilised in transesterification. Despite their high efficiency, these catalysts provide challenges for recycling and separation. Furthermore, the process of refining the resulting biodiesel can be resource-intensive, requiring substantial amounts of heat, water, and time, increasing the overall cost [16, 32].
However, there are several disadvantages to homogeneous catalysts, including their tendency to dissolve and neutralise, toxicity issues, restricted reusability, destructive influence on machinery, and high energy requirements during manufacturing—all of which add up to increased costs. These challenges can be mitigated by developing and implementing heterogeneous catalysts. Using heterogeneous catalysts dramatically improves biodiesel production. Notably, agricultural waste-derived calcium oxide (CaO) is a highly efficient base heterogeneous catalyst for biodiesel generation, with minimal solubility in both methanol and biodiesel, high catalytic activity, and low cost [33, 34].
Numerous optimisation studies have demonstrated that operational variables play a major role in the renewable fuel production process. Even with this understanding, it’s still very difficult to achieve the ideal reaction conditions to maximize output and maximize resource efficiency. According to [10, 35], a number of variables must be carefully examined, including temperature, catalyst concentration, reaction time, mixing intensity, and oil-to-methanol ratio. Several artificial intelligence technologies and machine learning techniques have been utilised to predict ideal conditions for biodiesel synthesis [36, 37]. This study focusses on the Taguchi orthogonal approach’s innovative use of RSM in the transesterification of Jatropha oil into renewable fuel. With a higher making of biodiesel from Jatropha oil, this sophisticated procedure is essential for increasing the productivity the transesterification technique. The purpose of the investigation is to enhance process parameters so that biodiesel may be produced sustainably from this renewable resource. The motivation behind utilizing Taguchi orthogonal approach is based on its well-known ability to conduct trials well while reducing the number of repeats essential to attain optimal outcomes. The research outputs boost comprehension of biodiesel production and give major perspectives for extensive industry implementations.
Jatropha oil (JCO) was chosen for the experiment and obtained in pure form from Agric-Energy Limited in Kano State, Nigeria, for biodiesel production purposes. Orange peels, cocoa pods, snail shells, and waste eggshells were sourced locally from Uselu Market and Efosxy Baking /Catering Services, both in Benin City, Edo State, Nigeria. High purity sodium hydroxide (NaOH) pellets, sulphuric acid (H\(_{2}\)SO\(_{4}\)), acetic acid, chloroform (CHCl\(_{3}\)), benzene, ethanol, methanol, distilled water, phenolphthalein indicator, sodium thiosulphate (Na\(_{2}\)S\(_{2}\)O\(_{3}\)), wij solution (ICl), potassium iodide (KI), and starch indicator were used for biodiesel production and analysis. Analytical standards were obtained from Luco Scientific Chemical Laboratory in Uselu, Benin City, Edo State, Nigeria.
To reduce moisture content, the catalyst precursors were carefully cleaned and sun-dried for two days. After that, they were dried in an oven set at 600\(\mathrm{^\circ}\)C for a whole day. Each catalyst was then pulverized using a grinding machine on an individual basis. First, a 1M sodium hydroxide (NaOH) solution was applied for 48 hours to an equal weight of eggshells and snail shells, which were used as alkaline precursors. Following this procedure, the mixture was dried for 24 hours at 50\(\mathrm{^\circ}\)C in an oven and calcined for 6 hours at 900\(\mathrm{^\circ}\)C in a box-oven. After cooling in a desiccator, the final products were kept in an airtight container to avoid absorbing moisture.
Likewise, phosphoric acid was applied to an equivalent weight of cocoa pods and orange peels, which functioned as acid precursors, for 48 hours before being baked at 50\(\mathrm{^\circ}\)C. Following three hours of carbonization at 450\(\mathrm{^\circ}\)C, the materials were taken out of the oven and set in a desiccator to cool before being kept dry in an airtight container.
To add SO3-ions to the acid precursor’s active sites, it was sulfonated at 150\(\mathrm{^\circ}\)C using a magnetic stirrer after it had cooled and dried. Then, using a wet impregnation procedure at 50\(\mathrm{^\circ}\)C, the acid and alkaline precursors were combined in various ratios of 1:1, 1:2, 2:1, and 3:2 to generate a heterogeneous catalyst. To attain the required particle size, the finished catalyst was sieved through a 500-micron sieve after being baked in an oven at 85\(\mathrm{^\circ}\)C and ground into a fine powder using a wooden mortar and pestle. Figure 1, shows the agrowastes for biofuntionalized catalyst.

The Jatropha seed oil (JSO) used in this study was refined in order to make it suitable for using in the manufacturing of biodiesel. Analyses were conducted on the physicochemical properties of the oil, which are reported in Table 1. These properties include density, iodine value, acid value, saponification value, water content, free fatty acid (FFA) content, and dynamic viscosity. According to recognized methods, these measurements were carried out.
| Properties (Unit) | Value |
|---|---|
| Acid value (mg KOH/g) | 11.04 |
| Saponification value (mg KOH/g) | 208 |
| FFA content (mg KOH/g) | 5.52 |
| Water content (wt. %) | 0.24 |
| Dynamic Viscosity (mPa.s) | 39.7 |
| Density (g/cm\(^{3}\)) | 0.899 |
| Iodine content (I\(_{2}\)g/100g) | 109 |
| Peroxide value (Meq/kg) | 9.6 |
| Std | Run | A:MeOH:Oil | B:Cat. Loading wt.% | C:Temperature deg. C | D:Time (min) |
|---|---|---|---|---|---|
| 8 | 1 | 15:1 | 2 | 60 | 120 |
| 9 | 2 | 15:1 | 3 | 65 | 60 |
| 4 | 3 | 12:1 | 1 | 65 | 120 |
| 5 | 4 | 12:1 | 2 | 70 | 60 |
| 7 | 5 | 15:1 | 1 | 70 | 90 |
| 3 | 6 | 9:1 | 3 | 70 | 60 |
| 2 | 7 | 9:1 | 2 | 65 | 90 |
| 6 | 8 | 12:1 | 3 | 60 | 90 |
| 1 | 9 | 9:1 | 1 | 60 | 60 |
| 8 | 1 | 15:1 | 2 | 60 | 120 |
| 9 | 2 | 15:1 | 3 | 65 | 60 |
The goal of the robust Taguchi orthogonal design approach is to lessen the impact of noise on trial outcomes. To determine the optimal values of different experimental parameters, this method considers the appropriateness of an orthogonal array and applies a signal-to-noise ratio (S/N) analysis. Enhancing the process’s robustness and consistency during optimisation is the primary advantage of the Taguchi technique, which also makes it simpler to pinpoint minor influencing aspects. RT (\(\mathrm{^\circ}\)C), RTtime(minutes), CL (weight percentage), and the MTOR (mol/mol) were all investigated in this study as independent variables. The optimal values for these variables were found by nine experimental runs using the Taguchi method (Table 2). In this way, the Taguchi technique reduces the number of tests needed and makes it easier to identify significant independent variables without negatively impacting the operating parameters [38]. This work used a 3-level, 4-factor Taguchi L9 design to build the experimental setup focused on the one-pot synthesis of biodiesel from jatropha seed oil. Eq. (1), according to [16]. \[\label{GrindEQ__1_} N\mathrm{=}\left(L\mathrm{-1}\right)P\mathrm{+1} , \tag{1}\] where ‘P’ is the number of design and control parameters selected, ‘L’ represents the number of levels. From Eq. (1), the entire experimental runs using Taguchi Orthogonal Array (OA) is 9. In order to calculate the number of experimental trials needed to produce biodiesel under specific combinations, the variables were entered into the software. For every trial, 50 grammes of Jatropha Seed Oil (JSO) were measured and applied to a 250 ml conical flask. The temperature, time, and weight of the bi-functional catalyst were estimated. The percentage of biodiesel generated upon washing was computed.
Analysis of the experiment results and the effects of the factors on the formation of Fatty Acid Methyl Ester were done using the signal-to-noise (S/N) ratio. Simply expressed, the S/N ratio is the logarithmic representation of the evaluated outcomes. It separates the findings into three groups: smaller-the-better, larger-the-better, and nominal-the-better. For this study, yield maximisation was the primary objective, hence the higher the S/N ratio, the better [39].
We analysed the experimental data using the S/N ratio to understand how different factors affect FAME yield. Using this ratio, the difference between the observed responses and the expected outcomes was computed in significant part [39] \[\label{GrindEQ__2_} \frac{S}{N}\mathrm{=-10}{log}_{\mathrm{10}}\left(\frac{\sum{\frac{\mathrm{1}}{{yi}^{\mathrm{2}}}}}{n}\right) , \tag{2}\] where y\(_{i}\) is the response value and n is the number of experimental runs. Eq. (2) is used for S/N ratio analysis to identify the best level for individual variable, which will maximize FAME yield. On the other hand, the challenge of elucidating the ways in which the different variables impact the percentage conversion of FFA is a drawback of this technique. ANOVA can be used to evaluate the individual variables in order to overcome this constraint [40]. To determine the importance of the selected model and the impact of each individual variable on the responses, the Fisher test (F-value) is employed within the context of the ANOVA investigation. In the meantime, figuring out the likelihood of getting the observed F-value depends heavily on the p-value. Each variable’s contribution and impact on biodiesel yield are determined using the sum of squares calculated from the model and individual variables. Eq. (3) can be used to determine each parameter’s contribution factor, which offers information about how it affects the process as a whole. \[\label{GrindEQ__3_} \mathrm{\%\ }contribution\mathrm{\ }of\mathrm{\ }factor\mathrm{=}\frac{{SS}_f}{{SS}_t}\mathrm{\times 100}, \tag{3}\] where \(SS_t\) stands for the sum of squares for all variables included in this framework, while \(SS_f\) refers to the sum of squares related to precise parameters. Enhancing the rate of conversion of free fatty acids (FFA) is the main objective of this analysis. As a result, the optimal variables were obtained in order to optimize the intended result. To show how the actual answers from experimental trials and the expected responses for all process variables relate to one another, a linear regression model can be utilized. This method is useful for confirming the accuracy of the model [16].
A bi-functionalized catalyst was an essential part of the transesterification transformation, which was performed in a 1000 ml batch reactor. The L9 arrangement, or Taguchi orthogonal array, served as the foundation for the experimental design. This process’s independent variables were the MTOR(mol/mol), RTime (minutes), CW (w/w), and RT(\(\mathrm{^\circ}\)C). With the goal of minimizing evaporation losses, the RT were carefully calibrated to be 50, 60, and 70 \(\mathrm{^\circ}\)C—close to the boiling point of methanol, which is 65 \(\mathrm{^\circ}\)C. For a duration of 60 to 120 minutes, near the boiling point of methanol to minimize losses, a mixture of catalyst and methanol was added to the Jatropha oil and continuously stirred with a magnetic stirrer within the temperature range of 60 to 70 \(\mathrm{^\circ}\)C. Using a reflux condenser to recycle the methanol vapor back into the reaction vessel allowed the system to retain the vapor. The mixture was put into a separating funnel after cooling from the reaction. Because of gravity, the glycerol and biodiesel in this instance split into two different layers, with the glycerol settling below and the less dense biodiesel floating on top. Opened the funnel’s tap, the biodiesel remained in the reactor while the glycerol was collected in a different vessel. Following filtering, the residual mixture was moved to a sanitized separating funnel and kept there over night. Slowly, glycerol accumulated at the funnel’s base. The condensed glycerol was removed and the leftover crude biodiesel was decanted once the settling process was finished. Once more purification and analysis were performed, this raw biodiesel was transferred into a glass container. To remove any remaining methanol and dissolved glycerol, the synthesized fuel was precisely extracted from the wastewater using a funnel and then properly cleaned with warm distilled water. The yield of biodiesel produced was evaluated through a given mathematical formula (4). \[\label{GrindEQ__4_} \mathrm{Percentage\ yield\ of\ biodiesel=}\frac{\mathrm{mass\ of\ biodiesel}}{\mathrm{mass\ of\ jatrpha\ seed\ oil}}\mathrm{\times 100} . \tag{4}\]
The primary challenge in converting pre-treated feedstock into FAME is the presence of pollutants in the oil, which can result in erratic reaction conditions and the generation of undesirable byproducts. These issues were addressed by carefully planning and fine-tuning the trial by modifying the process variables. Selecting the finest feedstock, employing purification procedures, and continuously monitoring the reaction’s progress were other essential strategies. By employing a systematic experimental methodology and optimising process factors, it is possible to significantly boost the yield and efficiency of manufacturing FAME from Jatropha oil. Figure 2 depicts the setup for the process of producing biodiesel.

Figure 3 visualizes the fourier transform infrared (FTIR) spectra of the bifunctional catalyst used in this study. Notable absorption peaks were detected at 3485.1 cm\(^{-1}\), 1801.2 cm\(^{-1}\), 1781.0 cm\(^{-1}\), 1511.8 cm\(^{-1}\), 1470.5 cm\(^{-1}\), 1103.3 cm\(^{-1}\), 878.9 cm\(^{-1}\), and 725.4 cm\(^{-1}\), as detailed in Table 3.
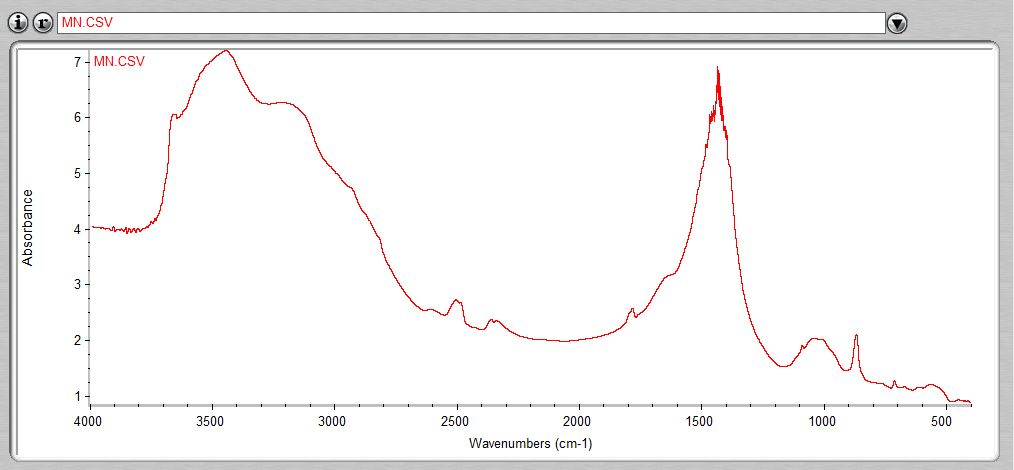
| Wave number (cm\(^{-1}\)) | Nature of frequency | Nature of bond | Functional group |
|---|---|---|---|
| 3485.1 | Strong and sharp | O-H(free) | Alcohols, phenols |
| 1470.5 | Medium | C-C (in-rings) | aromatics |
| 878.9 | Strong | C-H bending | Alkene |
| 1103.3 | Strong | C-O stretching | Alcohol |
| 1875.4 | Strong | S=O stretching | Sulfone |
| 1470.5 | Medium | C-H bending | Alkane |
| 1781.0 | Strong | C=O | cyclopentanone |
| 1511.8 | Medium | C=C | Cyclic akene |
Figure 4, present the results of the scanning electron microscopy (SEM) study of the bifunctional catalysts employed in this investigation. Various magnifications of 50, 80, 100, and 200 micrometers were utilised to capture the images, as shown in Figure 4(a), (b), (c), and (d), respectively. When examined more closely, the catalysts shown in Figure 4(a) and (b) showed a non-uniform pore size distribution with irregular shapes, similar to microscopic particle clusters. A large surface area that is beneficial for reactions and a large number of active catalytic sites that can improve the synthesis of biodiesel are indicated by the catalyst’s uneven surface structure and the buildup of tiny particles at its edges. Large pore spaces are characterised by deep channels, as seen in the SEM images in Figure 4(c) and (d). The sulfonation process that occurs during calcination is the cause of these larger holes, which are a significant part of the catalyst’s catalytic activity.

The experimental trials for the one-pot generation of biodiesel from Jatropha oil using, bi-functionalized catalyst were investigated using the 3-level, 4-factor Taguchi L9 design of experiment under response surface technique (design expert 7.0) (as shown in Table 4). displays the actual and anticipated values for Y1 (biodiesel yield percentage) and Y2 (FFA conversion, weight percentage) in the respective responses.
| Run Order | Actual Value | Predicted Value | Residual | Leverage | Internally Studentized Residuals | Externally Studentized Residuals | Cook’s Distance | Influence on Fitted Value DFFITS | Standard Order |
| 1 | 90.36 | 90.68 | -0.3189 | 0.556 | -0.466 | -0.415 | 0.054 | -0.464 | 8 |
| 2 | 95.20 | 94.34 | 0.8578 | 0.556 | 1.255 | 1.396 | 0.394 | 1.560 | 9 |
| 3 | 87.31 | 88.22 | -0.9089 | 0.556 | -1.330 | -1.541 | 0.442 | -1.723 | 4 |
| 4 | 80.23 | 79.05 | 1.18 | 0.556 | 1.733 | 3.005 | 0.751 | 3.359\(^(1)\) | 5 |
| 5 | 84.63 | 85.17 | -0.5389 | 0.556 | -0.788 | -0.743 | 0.155 | -0.831 | 7 |
| 6 | 76.67 | 77.32 | -0.6456 | 0.556 | -0.944 | -0.928 | 0.223 | -1.037 | 3 |
| 7 | 86.54 | 86.49 | 0.0511 | 0.556 | 0.075 | 0.065 | 0.001 | 0.072 | 2 |
| 8 | 84.28 | 84.56 | -0.2756 | 0.556 | -0.403 | -0.356 | 0.041 | -0.398 | 6 |
| 9 | 83.42 | 82.83 | 0.5944 | 0.556 | 0.870 | 0.836 | 0.189 | 0.935 | 1 |
Quantitative analysis was conducted to analyse the impacts of process variables on biodiesel synthesis. Using the experimental data, we performed multiple regression analysis to find regression model equations that accurately depict the relationship between these factors. The three main variables that are utilized to anticipate the results are correlated by the mathematical equations Eqs. (5) and (6) that are obtained from the Taguchi method.
\[ Y_{1}(Biodiesel yield, wt\%) = +85.40 – 3.19A[2] – 1.46A[2] + 0.6156C[1] + 4.28C[2],\label{GrindEQ__5_}\ \tag{5}\] \[Y_{2}(FFA conversion, wt\%) = +97.16 – 0.8533A[1]+ 0.0767A[2] + 0.6500C[1] + 0.1533C[2]\notag\ \tag{6}\] \[ – 0.3517D[1] – 0.1967D[2].\label{GrindEQ__6_} \]
The MTOR at the first and second levels is represented by A[1] and A[2], the temperatures are represented by C[1] and C[2], and the corresponding RTime are represented by D[1] and D[2]. The proportionate impact of each element on the yield can be ascertained by comparing the regression equation’s coefficient. With the help of the equation defined in terms of real factors, it is feasible to predict the response for particular levels of each element. Only essential variables—the MTOR, RT, and RTime are included in the model equations. The catalyst loading and other unimportant factors were taken out of the model equations.
| Std | Run | A:MeOH:Oil | B:Cat. Loading (wt.%) | C:Temperature (0C) | D:Time (min) | JOME yield (%) | FFA conversion (%) |
| 8 | 1 | 15:1 | 2 | 60 | 120 | 90.36 | 99.08 |
| 9 | 2 | 15:1 | 3 | 65 | 60 | 95.2 | 97.75 |
| 4 | 3 | 12:1 | 1 | 65 | 120 | 87.31 | 98 |
| 5 | 4 | 12:1 | 2 | 70 | 60 | 80.23 | 96.01 |
| 7 | 5 | 15:1 | 1 | 70 | 90 | 84.63 | 96.99 |
| 3 | 6 | 9:1 | 3 | 70 | 60 | 76.67 | 95.18 |
| 2 | 7 | 9:1 | 2 | 65 | 90 | 86.54 | 96.2 |
| 6 | 8 | 12:1 | 3 | 60 | 90 | 84.28 | 97.71 |
| 1 | 9 | 9:1 | 1 | 60 | 60 | 83.42 | 96.65 |
| S/N | Source | Sum of Squares (SS) | Degrees of Freedom | Mean Square | F-value | p-value | Contribution factors (%) | |
| 1 | Model | 11.39 | 6 | 1.90 | 171.92 | 0.0058 | significant | |
| 2 | A – MeOH:Oil | 3.36 | 2 | 1.68 | 151.99 | 0.0065 | 29.49 | |
| 3 | C – Temperature | 2.69 | 2 | 1.35 | 121.96 | 0.0081 | 23.62 | |
| 4 | D – Time | 0.7621 | 2 | 0.3810 | 34.51 | 0.0282 | 6.69 | |
| 5 | Residual | 0.0221 | 2 | 0.0110 | ||||
| 6 | Cor Total | 11.41 | 8 |
The projected Y1 (% biodiesel yield) and Y2 (FFA conversion, w%) produced from the methanolysis transformation utilizing the L9 Taguchi orthogonal array technique are shown in Table 5. The importance of each reaction parameter and its impact on the results, as well as other important quantitative elements that demonstrate the relationship—or lack thereof—between the experimentally acquired data and the mathematically predicted outcomes, are determined through the use of the ANOVA analysis. Eqs. (4) and (5) of the regression model are utilized to express this relationship. Tables 6 and 7 demonstrate how the ANOVA analysis provides for the model. Furthermore, Table 8 presents the R², adjusted R², and anticipated R² values, obtained from the ANOVA evaluation. The models are deemed appropriately fitted, as evidenced by the Model F-value of 171.92 for both the biodiesel yield and the FFA conversion. It is interesting that the F-value of this kind originating from random variation is merely 0.58%. Model terms having a p-value less than 0.1000 are considered significant. The MTOR, RT, and RTime are the only critical elements included in the model equations; catalyst loading, an inconsequential influence, was left out. As a result, it is established that theMTOR, RT, and RTime are relevant parameters, but catalyst loading is not. Additionally, the calculated R2 values of 0.9821 for biodiesel output and 0.9981 for free fatty acid (FFA) conversion demonstrate that an incredible 99.81% and 98.21% of the overall variations were explained by the operational parameters under examination. Only 0.19% and 1.79% of the variations remain unaccounted for by the models. The R2 values’ proximity to one implies a robust linear relationship and a well-fitting model. The updated R² values for the FFA conversion and biodiesel yield were found to be 0.9923 and 0.9641, respectively, whereas the predicted R² values for the FFA conversion and biodiesel yield models were 0.9539 and 0.9091. A signal-to-noise ratio’s appropriate accuracy, which is a vital statistic, is regarded acceptable when it is higher than 4. According to Table 8, the study’s proper precision values of 42.9835 for FFA conversion and 22.2785 for biodiesel output reveal a suitable signal. The model’s ability to accurately forecast the dependent variables and effectively maximize the yield of fatty acid methyl ester (FAME) and FFA conversion is further evidenced by these sufficient precision scores. Additionally, the model accurately predicted the optimal parameters; the biodiesel yield and FFA conversion standard deviations were 0.1083% and 1.03, respectively, with coefficient variances of 1.20% and 0.1083%.
| S/N | Source | Sum of Squares | Degrees of Freedom | F-value | p-value | Contribution factors (%) | |
| 1 | Model | 11.39 | 6 | 171.92 | 0.0058 | significant | |
| 2 | A-MeOH: Oil | 3.36 | 2 | 151.99 | 0.0065 | 29.49 | |
| 3 | C-Temperature | 2.69 | 2 | 121.96 | 0.0081 | 23.62 | |
| 4 | D-Time | 0.7621 | 2 | 34.51 | 0.0282 | 6.69 | |
| 5 | Residual | 0.0221 | 2 | ||||
| 6 | Cor Total | 11.41 | 8 |
| S/N | Parameter | FFA conversion Value | Biodiesel Yield Value |
|---|---|---|---|
| 1. | Std. Dev. | 0.1051 | 1.03 |
| 2. | Mean | 97.06 | 85.40 |
| 3. | C.V. % | 0.1083 | 1.20 |
| 4. | R² | 0.9981 | 0.9821 |
| 5. | Adjusted R² | 0.9923 | 0.9641 |
| 7. | Predicted R² | 0.9539 | 0.9091 |
| 8. | Adequate Precision | 42.9835 | 22.2785 |
Experimental results can be used to determine the impacts of several practical variables on the synthesis of biodiesel from Jatropha oil. The production of biodiesel is significantly influenced by the MTOR, the RT, and the RTime, as demonstrated by the ANOVA and the contributions of individual variables (Tables 6 and 7). Thus, a comprehensive analysis of these factors has been carried out. On the other hand, because catalyst loading has little effect on the conversion of FFA and the yield of biodiesel, its effects have not been thoroughly examined. The catalyst loading that was chosen was within what is usually thought to be the best range for yield conversion. Even though the catalyst is crucial to the reaction process, it was determined that in this instance, the catalyst had little effect because the yield of FAME varied very little within the designated catalyst range.
Studies show that the MTOR is an important factor in affecting the Jatropha biodiesel output (Tables 6 and 7). Under particular circumstances, the maximum yield of FAME was produced at a CL (1wt%), RT (60 \(\mathrm{^\circ}\)C), and RTime(60minutes). The changes in FFA conversion and biodiesel yield at various MTOR are shown in Figures 5 and 6. These numbers show that higher biodiesel production and FFA conversion occur when the MTOR is increased from 9:1 to 12:1 and then from 12:1 to 15:1. This supports the idea that biodiesel synthesis from Jatropha seed oil (JSO) is favored by an increased methanol supply. On the other hand, too much methanol might dilute the reaction environment, making it more difficult for reactant molecules to interact with the catalyst and slowing down the rate of reaction. As a result, careful management of methanol levels is necessary to avoid dilution from impairing the catalyst’s efficiency. Furthermore, an excessively high MTOR could prevent the separation of glycerol, which would reduce the output of biodiesel [40, 41]. However, Table 6 indicates that this component only contributes 29.49% to the whole biodiesel manufacturing process, demonstrating that the methanol-to-oil ratio is still an essential step in the biodiesel synthesis process even at low levels of optimization.
Significance of temperature in a heterogeneous-catalyzed reaction is paramount and should not be overlooked. High temperatures generally impair these processes, frequently resulting in catalyst deactivation, reduced product quality, and reactant losses. In an inquiry examining the influence of temperature on biodiesel synthesis, several critical factors were held constant: the reaction duration was established at 60 minutes, the methanol-to-oil molar ratio was preserved at 9:1, and the catalyst loading was fixed at 1 wt%. Figures 7 and 8 depict the fluctuations in biodiesel output and free fatty acid (FFA) conversion across various temperatures. The tested temperature range was 60\(\mathrm{^\circ}\)C to 65\(\mathrm{^\circ}\)C, with 65\(\mathrm{^\circ}\)C determined as the best temperature for maximizing reaction rate and biodiesel yield. Conversely, an elevation in temperature from 65\(\mathrm{^\circ}\)C to 70\(\mathrm{^\circ}\)C led to a substantial decline in biodiesel production. The drop is due to temperatures surpassing 65\(\mathrm{^\circ}\)C, which might surpass methanol’s boiling point during the reaction, leading to the vaporization of methanol—the reactant—and consequently disrupting the desired methanol-to-oil molar ratio Figure 5 demonstrates that a peak conversion rate of 96.66% was attained at 60\(\mathrm{^\circ}\)C. The transesterification reaction is endothermic, making it significant that increasing the temperature promotes the reaction’s forward progression [40, 41]. Moreover, elevated temperatures increase the collision frequency among the three phases, hence enhancing miscibility [42]. Nonetheless, additional temperature elevations beyond 60\(\mathrm{^\circ}\)C and 65\(\mathrm{^\circ}\)C result in the vaporization of methanol, hence diminishing the methanol content in the reaction mixture. Moreover, the results in Table 6 indicate that temperature constitutes merely 23.62% of the overall process contribution, suggesting that even at the fundamental optimization level, reaction temperature is crucial in biodiesel production. Thus, the ideal temperature for optimizing biodiesel yield is identified at 65\(\mathrm{^\circ}\)C, establishing it as the upper threshold for efficient biodiesel production.
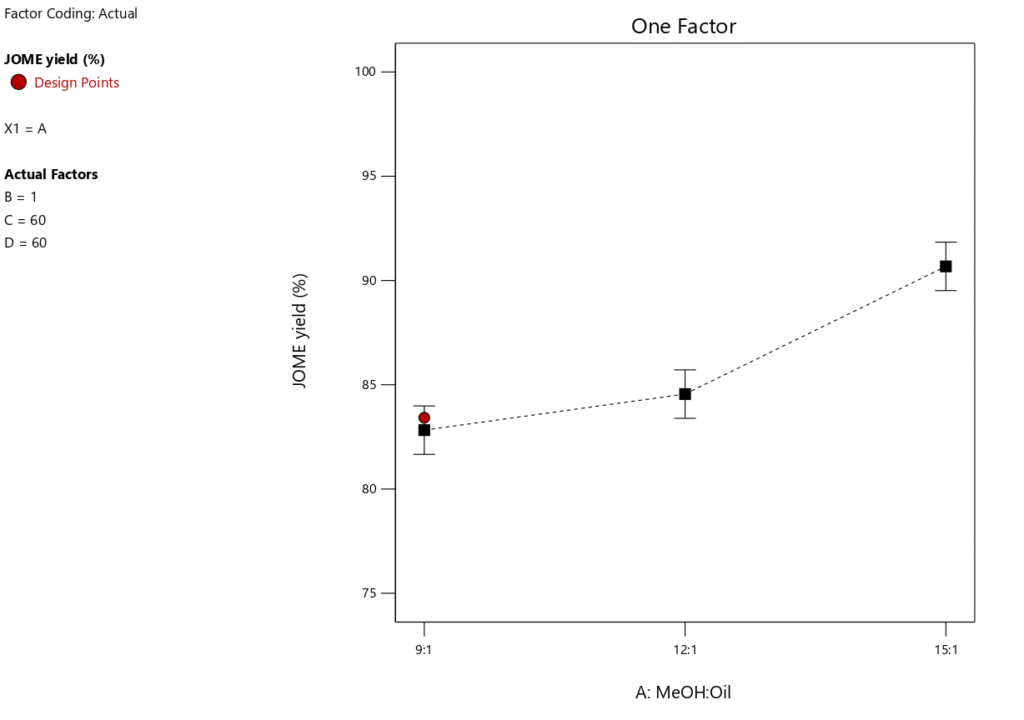
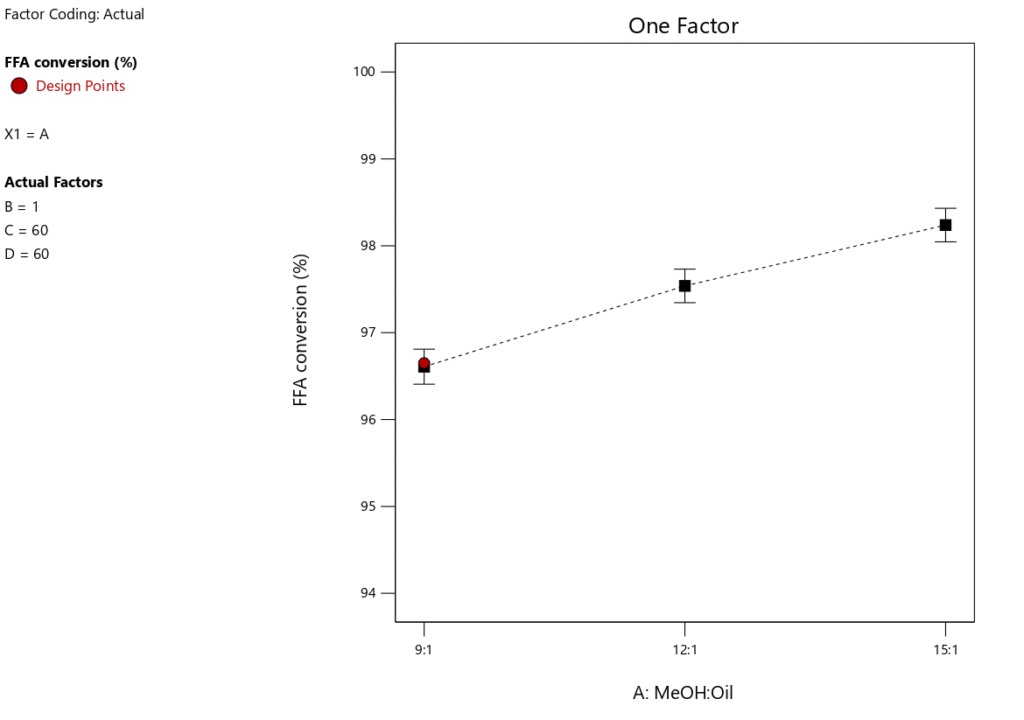
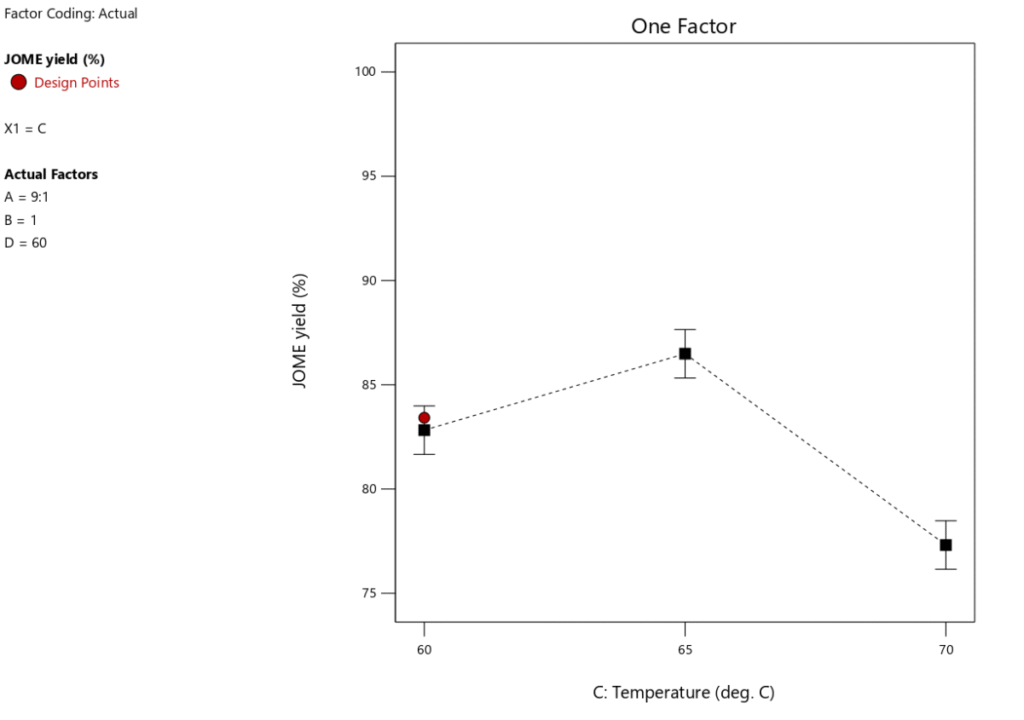
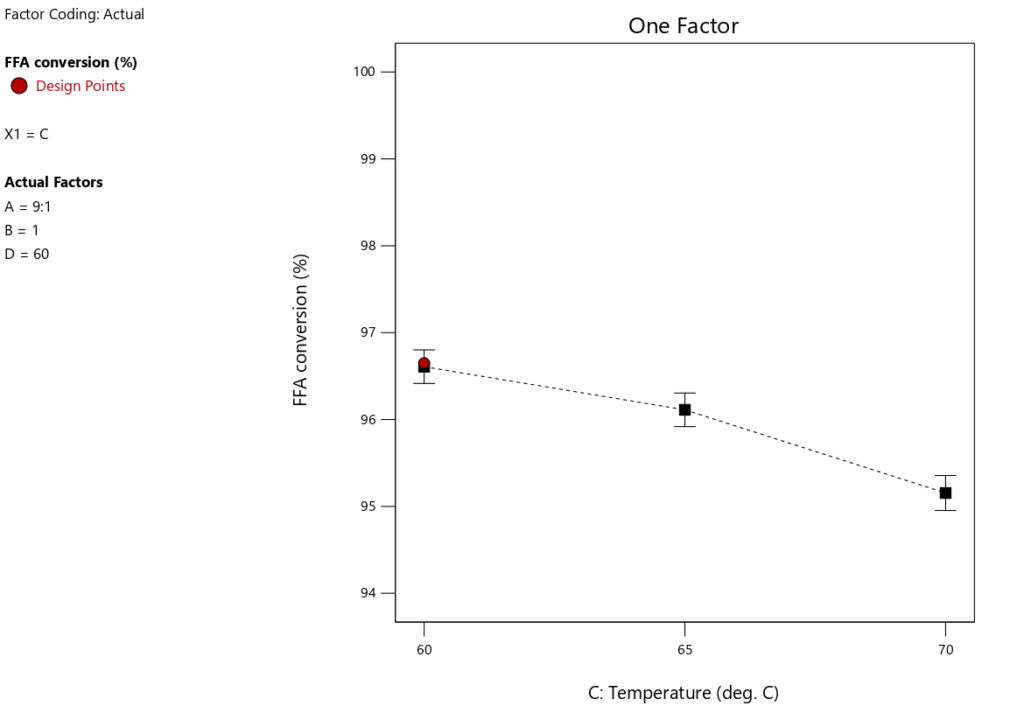
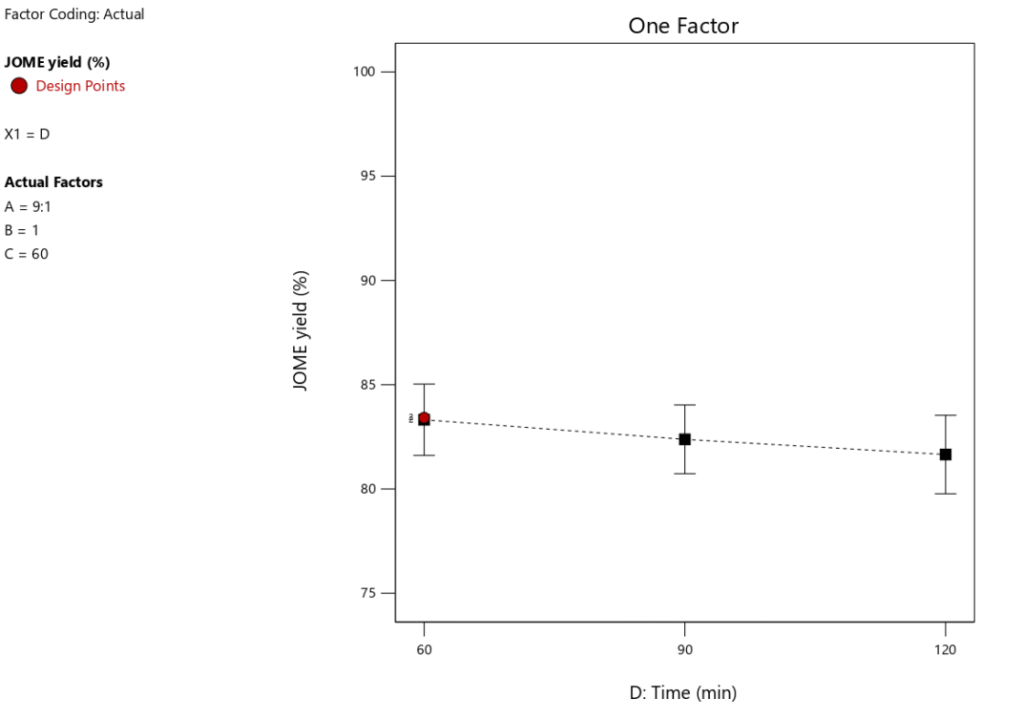
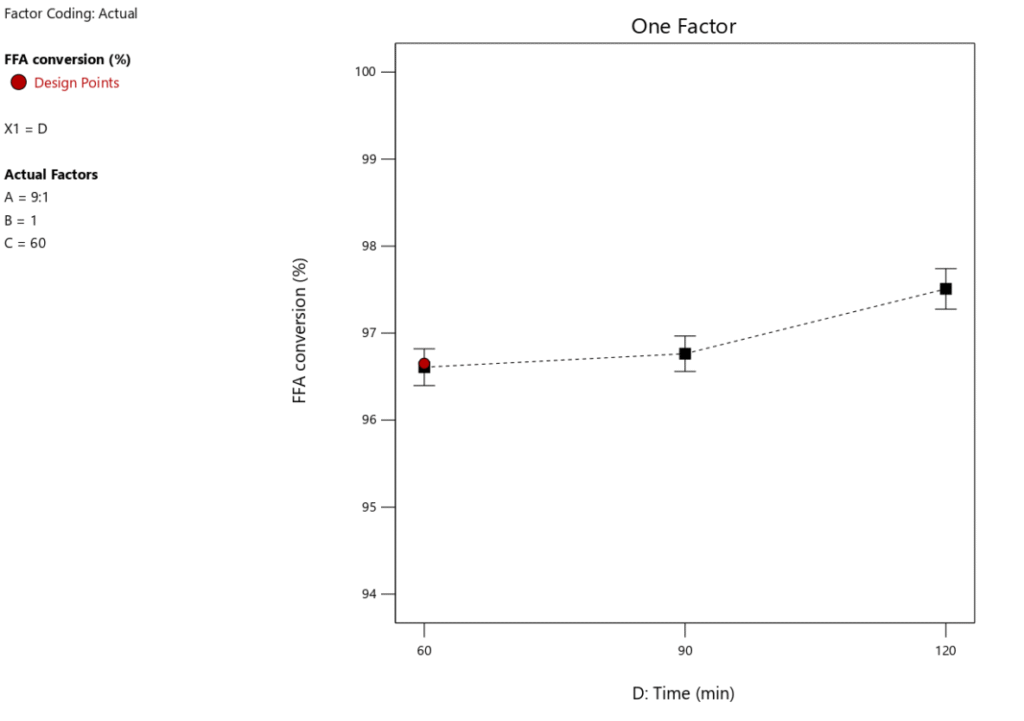
One key feature of biodiesel production to take into account is RTime. An optimal RTime is necessary to produce a high biodiesel yield. Figure 9 illustrates how RTime affects the yield of biodiesel. The experiment’s other key parameters included a catalyst loading of 1 wt.%, a MTORof 9:1, and a RTof 60\(\mathrm{^\circ}\)C. Figure 10 shows that as RTime increases, the yield of biodiesel tends to decrease, suggesting that the optimal reaction duration for maximum biodiesel yield is 60 minutes. Figure 10 illustrates how the conversion of FFA increases dramatically as the reaction time increases from 60 to 90 minutes. As the reaction time rises from 90 to 120 minutes, this pattern continues. Therefore, a longer reaction time improves the conversion rate of FFA and encourages a higher percentage output of biodiesel. However, as Table 6 illustrates, this variable only accounts for 6.69% of the process as a whole, meaning its influence is less than that of the other factors our study examined. This indicates that even at low optimization levels, response time remains a crucial component of the biodiesel synthesis process.
The one-pot JSO biodiesel production method needed to be refined in order to determine the optimal parameters that can increase the yield of JSO biodiesel and encourage conversion of FFA. The variables were changed using numerical optimization while adhering to the specified limitations because temperature and catalyst loading were important factors in the conversion of FFA to biodiesel, but the length of the reaction and the ratio of methanol to oil had a significant impact on the production of biodiesel. The optimal parameters in this investigation were determined to be a MTOR of 15:1, a catalyst loading of 3 Wt.%, a RT of 65\(\mathrm{^\circ}\)C, and a RTime of 60 minutes. These parameters produced a biodiesel yield of 95.2 weight percent and an FFA conversion rate of 97.75%. Table 9 displays the biodiesel output percentages and FFA conversion rates for the various trials.
| Std | Run | A: MeOH:Oil | B: Cat. Loading wt.% | C: Temperature \(^0\)C | D: Time Min | JOME yield % | FFA conversion % |
| 8 | 1 | 15:1 | 2 | 60 | 120 | 90.36 | 99.08 |
| 9 | 2 | 15:1 | 3 | 65 | 60 | 95.2 | 97.75 |
| 4 | 3 | 12:1 | 1 | 65 | 120 | 87.31 | 98 |
| 5 | 4 | 12:1 | 2 | 70 | 60 | 80.23 | 96.01 |
| 7 | 5 | 15:1 | 1 | 70 | 90 | 84.63 | 96.99 |
| 3 | 6 | 9:1 | 3 | 70 | 60 | 76.67 | 95.18 |
| 2 | 7 | 9:1 | 2 | 65 | 90 | 86.54 | 96.2 |
| 6 | 8 | 12:1 | 3 | 60 | 90 | 84.28 | 97.71 |
| 1 | 9 | 9:1 | 1 | 60 | 60 | 83.42 | 96.65 |
The study investigated catalyst’s economic feasibility for manufacturing industrial biodiesel as well as its reusability. Using a one-pot procedure, biodiesel was made from Jatropha Seed Oil (JSO) in a number of experimental trials. Following each cycle, the results were analyzed along with the catalyst recovery rates to ascertain the impact of the catalyst’s reusability on the amount of biodiesel generated and the conversion of FFA.
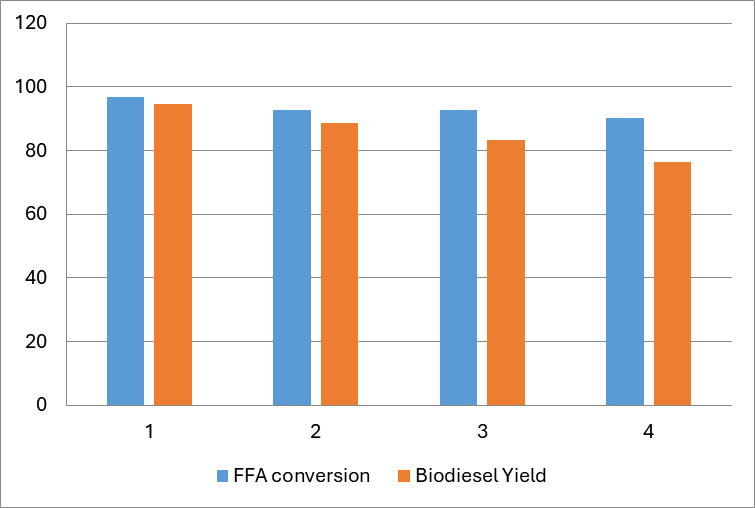
As seen in Figure 11, the proportion of reusable catalyst at the end of each cycle was recorded. 94.66% of the FFA conversion and 94.58% of the biodiesel output were attained when the catalyst was first utilized. Beginning with the second run, the biodiesel yield dropped to 88.69% and the FFA conversion to 92.86%. The biodiesel yield decreased even more to 83.27% in the third cycle, while the FFA conversion remained relatively unchanged at 92.7% from the prior run. Figure 11 illustrates how the biodiesel yield dropped even more precipitously in the fourth cycle, at 76.38%. However, the FFA conversion rate only dropped to 90.28%. One possible explanation for the decline in biodiesel production seen with each succeeding cycle is the decrease in catalytic activity brought on by the blocking of active sites on the heterogeneous catalyst.
This work refined a novel one-pot transesterification method based on bio-functional catalysts to yield FAME from Jatropha oil. Through the application of TOA, the investigation was able to optimise several process parameters and gain valuable insights into how these factors impact the final product. The results showed how well the Taguchi technique enhances the methanolysis process.
In order to create biodiesel, the study also suggested that waste materials from bioprocessing could be used to create a practical catalyst that could simultaneously transesterify and esterify oils with a high FFA content. Ideal conditions included a 15:1MTOR, 3 wt.% catalyst loading, a 60-minuteRTime, and a RT of 65\(\mathrm{^\circ}\)C. Under these conditions, a conversion rate of 95.20% was made. A 15:1 MTOR, 2 wt.% catalyst loading, 120-minuteRTime, and 60\(\mathrm{^\circ}\)C RT were all necessary to achieve an FFA conversion rate of 96.66%. The catalyst’s FFA conversions of 90.28% and 76.38%, respectively, showed that it was highly reusable even after four uses.
The methanol to oil ratio was the most significant of the three significant factors, according to the analysis of variance (ANOVA), which had a p-value of 0.0065 and a contribution factor of 29.49. The ANOVA results for FAME yield revealed that both the R\(^{2}\) and adjusted R\(^{2}\) values supported the models’ consistency and reliability against expected values, confirming the models’ acceptability and effectiveness.
Because their equations predicted FAME yields with a respectable level of accuracy and precision, the models developed by this study were significant. Using the Taguchi method, which required only nine experimental runs, proved to be cost-effective and time-efficient. The effectiveness of methanolysis has been accelerated thanks in large part to this innovative technique.
In addition, the study emphasised the necessity of environmentally friendly production techniques and offered opportunities for additional change through the optimisation of process variables. Further research and development in the biodiesel industry are made possible by the implementation of this strategy. Crucially, the biodiesel produced demonstrated its economic viability by meeting ASTM D6751 and EN 14214 requirements.
In conclusion, a great advance has been made using the unique strategy of modeling and optimising the Taguchi orthogonal method’s transesterification of Jatropha oil into fatty acid methyl ester. This unique approach improves the production of biodiesel in terms of quality, quantity, and utility. Researchers can overcome the limitations of conventional techniques by utilizing this advanced optimization strategy, opening the door for more environmentally friendly biodiesel production procedures.
| ANOVA | Analysis of variance |
| CW | Catalyst weight |
| CL | Catalyst loading |
| CV | Coefficient of variance |
| DOE | Design of experiment |
| FAME | Fatty acid methyl ester |
| FFA | Free fatty acid |
| JCO | Jatropha curcus oil |
| JSO | Jatropha seed oil |
| MTOR | Methanol to oil ratio |
| R\(^{2}\) | Coefficient of determination |
| RTime | Reaction time |
| RT | Reaction temperature |
| R\({}_{adj}\)\(^{2}\) | Adjusted Coefficient of determination |
| RSM | Response surface methodology |
| TOA | Taguchi orthogonal approach |
| SS | Total and individual sums of squares |
| F-value | Fisher’s test |
| p-value | Probability of occurrence |
Singh, D., Sharma, D., Soni, S. L., Sharma, S., Sharma, P. K., & Jhalani, A. (2020). A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel, 262, 116553.
Wan, L., Liu, H., & Skala, D. (2014). Biodiesel production from soybean oil in subcritical methanol using MnCO3/ZnO as catalyst. Applied Catalysis B: Environmental, 52, 352–359.
Javed, F., Rizwan, M., Asif, M., Ali, S., Aslam, R., Akram, M. S., Zimmerman, W. B., & Rehman, F. (2022). Intensification of biodiesel processing from waste cooking oil, exploiting cooperative microbubble and bifunctional metallic heterogeneous catalysis. Bioengineering, 9(10), 533.
Gutiérrez-López, A. N., Mena-Cervantes, V. Y., García-Solares, S. M., Vazquez-Arenas, J., & Hernández-Altamirano, R. (2021). NaFeTiO4/Fe2O3–FeTiO3 as heterogeneous catalyst towards a cleaner and sustainable biodiesel production from Jatropha curcas L. oil. Journal of Cleaner Production, 304, 127106. https://doi.org/10.1016/j.jclepro.2021.127106
Corro, G., Sánchez, N., Pal, U., Cebada, S., & Fierro, J. L. G. (2017). Solar-irradiation driven biodiesel production using Cr/SiO2 photocatalyst exploiting cooperative interaction between Cr\(^{6+}\) and Cr\(^{3+}\) moieties. Applied Catalysis B: Environmental, 203, 43–52.
Voloshin, R. A., Rodionova, M. V., Zharmukhamedov, S. K., Veziroglu, T. N., & Allakhverdiev, S. I. (2016). Biofuel production from plant and algal biomass. International Journal of Hydrogen Energy, 41(39), 17257–17273.
Amenaghawon, A. N., Evbarunegbe, N. I., & Obahiagbon, K. (2021). Optimum biodiesel production from waste vegetable oil using functionalized cow horn catalyst: A comparative evaluation of some expert systems. Cleaner Engineering and Technology, 4, 100184.
Mahlia, T. M. I., Syazmi, Z. A. H. S., Mofijur, M., Abas, A. P., Bilad, M. R., Ong, H. C., & Silitonga, A. S. (2020). Patent landscape review on biodiesel production: Technology updates. Renewable and Sustainable Energy Reviews, 118, 109526.
Sai, B. A., Subramaniapillai, N., Mohamed, M. S. B. K., & Narayanan, A. (2020). Optimization of continuous biodiesel production from rubber seed oil (RSO) using calcined eggshells as heterogeneous catalyst. Journal of Environmental Chemical Engineering, 8(1), 103603.
Ewunie, G. A., Morken, J., Lekang, O. I., & Yigezu, Z. D. (2021). Factors affecting the potential of Jatropha curcas for sustainable biodiesel production: A critical review. Renewable and Sustainable Energy Reviews, 137, 110500.
Pooja, S., Anbarasan, B., Ponnusami, V., & Arumugam, A. (2021). Efficient production and optimization of biodiesel from kapok (Ceiba pentandra) oil by lipase transesterification process: Addressing positive environmental impact. Renewable Energy, 165, 619–631.
Mansir, N., Taufiq-Yap, Y. H., Rashid, U., & Lokman, I. M. (2017). Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Conversion and Management, 141, 171–182.
Jamil, F., Kumar, P. S. M., Al-Haj, L., Myint, M. T. Z., & Ala’a, H. (2021). Heterogeneous carbon-based catalyst modified by alkaline earth metal oxides for biodiesel production: Parametric and kinetic study. Energy Conversion and Management: X, 10, 100047.
Etim, A. O., Musonge, P., & Eloka-Eboka, A. C. (2020). Effectiveness of biogenic waste-derived heterogeneous catalysts and feedstock hybridization techniques in biodiesel production. Biofuels, Bioproducts and Biorefining, 14(3), 620–649.
Bashir, M. A., Wu, S., Zhu, J., Krosuri, A., Khan, M. U., & Aka, R. J. N. (2022). Recent development of advanced processing technologies for biodiesel production: A critical review. Fuel Processing Technology, 227, 107120.
Aseibichin, C., Ulakpa, W. C., Omenogor, I., Doyah, E., Olaseinde, A. A., Anakpoha, O. C., & Karuppannan, S. (2024). Modeling and optimization of transesterification of Jatropha oil to fatty acid methyl ester: Application of response surface methodology (CCD) and Taguchi orthogonal method. RSC Advances, 14(17), 11784–11796.
Akhabue, C. E., Osa-Benedict, E. O., Oyedoh, E. A., & Otoikhian, S. K. (2020). Development of a bio-based bifunctional catalyst for simultaneous esterification and transesterification of neem seed oil: Modeling and optimization studies. Renewable Energy, 152, 724–735.
Olubunmi, B. E., Karmakar, B., Aderemi, O. M., Auta, M., & Halder, G. (2020). Parametric optimization by Taguchi L9 approach towards biodiesel production from restaurant waste oil using Fe-supported anthill catalyst. Journal of Environmental Chemical Engineering, 8(5), 104288.
Pourkarimi, S., Hallajisani, A., Alizadehdakhel, A., & Nouralishahi, A. (2019). Biofuel production through micro- and macroalgae pyrolysis—A review of pyrolysis methods and process parameters. Journal of Analytical and Applied Pyrolysis, 142, 104599.
Okolie, J. A., Escobar, J. I., Umenweke, G., Khanday, W., & Okoye, P. U. (2022). Continuous biodiesel production: A review of advances in catalysis, microfluidic and cavitation reactors. Fuel, 307, 121821.
Brethauer, S., & Studer, M. H. (2015). Biochemical conversion processes of lignocellulosic biomass to fuels and chemicals—A review. Chimia, 69(10), 572–572.
Okolie, J. A., Epelle, E. I., Tabat, M. E., Orivri, U., Amenaghawon, A. N., Okoye, P. U., & Gunes, B. (2022). Waste biomass valorization for the production of biofuels and value-added products: A comprehensive review of thermochemical, biological and integrated processes. Process Safety and Environmental Protection, 159, 323–344.
Wisdom, C. U., Ruth, O. U., Egwunyenga, M. C., & Egbosiuba, T. C. (2022). Transesterification of non-edible oil and effects of process parameters on biodiesel yield. Cleaner Waste Systems, 3, 100047.
Singh, D., Sharma, D., Soni, S. L., Sharma, S., & Kumari, D. (2019). Chemical compositions, properties, and standards for different generation biodiesels: A review. Fuel, 253, 60–71.
Tabatabaei, M., Aghbashlo, M., Dehhaghi, M., Panahi, H. K. S., Mollahosseini, A., Hosseini, M., & Soufiyan, M. M. (2019). Reactor technologies for biodiesel production and processing: A review. Progress in Energy and Combustion Science, 74, 239–303.
Najjar, R., & Heidari, S. (2018). Modified diesel prepared by stabilization of water as nanodroplets in diesel/colza oil blend: Study of phase behavior and affecting parameters. Fuel, 214, 497–504.
Hussain, F., Alshahrani, S., Abbas, M. M., Khan, H. M., Jamil, A., Yaqoob, H., & Munir, M. (2021). Waste animal bones as catalysts for biodiesel production; a mini review. Catalysts, 11(5), 630.
Okpalaeke, K. E., Ibrahim, T. H., Latinwo, L. M., & Betiku, E. (2020). Mathematical modeling and optimization studies by Artificial neural network, genetic algorithm and response surface methodology: A case of ferric sulfate–catalyzed esterification of Neem (Azadirachta indica) seed oil. Frontiers in Energy Research, 8, 614621.
Galvan, D., Cremasco, H., Mantovani, A. C. G., Bona, E., Killner, M., & Borsato, D. (2020). Kinetic study of the transesterification reaction by artificial neural networks and parametric particle swarm optimization. Fuel, 267, 117221.
Akhabue, C. E., & Okwundu, O. S. (2019). Monitoring the transesterification reaction of castor oil and methanol by ultraviolet visible spectroscopy. Biofuels, 10(6), 729–736.
Awogbemi, O., Von Kallon, D. V., & Aigbodion, V. S. (2021). Trends in the development and utilization of agricultural wastes as heterogeneous catalyst for biodiesel production. Journal of the Energy Institute, 98, 244–258.
Wisdom, C. U., Cyrus, A., Ohiri, A. C., Ayodeji, A. O., Eyide, O., Erhinyodavwe, O., & Ijara, M. A. (2024). Nano-CaO as a heterogeneous catalyst for biodiesel synthesis by transesterification of Jatropha oil. Journal of Trace Elements and Minerals, 9, 100183.
Jume, B. H., Parandi, E., Nouri, M., Aghel, B., Gouran, A., Nodeh, H. R., & Rezania, S. (2023). Optimization of microreactor-assisted transesterification for biodiesel production using bimetal zirconium-titanium oxide doped magnetic graphene oxide heterogeneous nanocatalyst. Chemical Engineering and Processing-Process Intensification, 191, 109479.
Aisien, F. A., Uwadiae, K. O., & Aisien, E. T. (2023). Process optimization for blended waste frying oil in biodiesel production using CaO derived from African periwinkle shell catalyst through response surface methodology. Sustainable Chemistry for the Environment, 4, 100042.
Wisdom, C. U., Ude, C. N., & Ruth, O. U. (2019). Optimization, kinetics and thermodynamics study of transesterification of neem oil using heterogeneous catalyst derived from waste. International Journal of Emerging Engineering Research and Technology, 7(9), 7–18.
Okonkwo, C. P., Ajiwe, V. I. E., Mmaduakor, E. C., & Nwankwo, N. V. (2022). Modelling and optimization of biodiesel production process parameters from Jansa seed oil (Cussonia bateri) using artificial neural network. American Journal of Applied Chemistry, 10(1), 7–14.
Okonkwo, C. P., Ajiwe, V. I. E., Mmaduakor, E. C., & Nwankwo, N. V. (2022). Modelling and optimization of biodiesel production process parameters from Jansa seed oil (Cussonia bateri) using artificial neural network. American Journal of Applied Chemistry, 10(1), 7–14.
Shojaei, S., Shojaei, S., Band, S. S., Farizhandi, A. A. K., Ghoroqi, M., & Mosavi, A. (2021). Application of Taguchi method and response surface methodology into the removal of malachite green and auramine-O by NaX nanozeolites. Scientific Reports, 11(1), 16054.
Omojola, A., Inambao, F. L., & Onuh, E. I. (2019). Modelling and optimization of transesterification of waste sunflower oil to fatty acid methyl ester: A case of response surface methodology vs Taguchi orthogonal approach. International Journal of Engineering Research and Technology, 12(12), 2346–2361.
Karmakar, B., Dhawane, S. H., & Halder, G. (2018). Optimization of biodiesel production from castor oil by Taguchi design. Journal of Environmental Chemical Engineering, 6(2), 2684–2695.
Dhawane, S. H., Kumar, T., & Halder, G. (2016). Biodiesel synthesis from Hevea brasiliensis oil employing carbon supported heterogeneous catalyst: Optimization by Taguchi method. Renewable Energy, 89, 506–514.
Thinnakorn, K., & Tscheikuna, J. (2014). Transesterification of palm olein using sodium phosphate impregnated on an alumina support. Applied Catalysis A: General, 484, 122–133.