Background and objectives: Postoperative nausea and vomiting (PONV) are still one of the most common and unpleasant side effects following surgeries under general anesthesia, especially in patients receiving opioids. The primary purpose of this study was to compare Zofran and oxycodone versus Zofran and morphine for the prevention of postoperative nausea and vomiting.
Subjects and methods: A prospective, monocentric clinical trial study was conducted at Mount Lebanon Hospital (MLH) between November 2018 and November 2021. After getting approval from the ethical committee, 237 patients were enrolled on a ratio of 1 to 2 in two groups: the first receiving Oxycodone 5 mg IVP with Zofran 4 mg IVD and the second receiving Morphine 5 mg IVP with Zofran 4mg IVD. Pearson’s Chi-square test and Fisher’s exact test were used to checking for the groups’ differences.
Results: Morphine and Oxycodone had a similar analgesic effect. The use of Zofran lowered PONV incidence rates in both groups. A statistically significant (p-value = 0.047) lower nausea and vomiting incidence in the Morphine group (2.5%) was noted compared to the Oxycodone group (8.8\% incidence). Only 10% of Oxycodone group patients and 8.9% of Morphine group patients had moderate nausea and vomiting, and none of the group’s patients had severe nausea and vomiting. Surgery duration, gender, age, smoking, BMI, pain level, and treatment group were not statistically associated with the severity of NV.
Conclusion: With the same analgesic effect, Zofran injection seemed to lower PONV incidence in both groups, even though the Morphine group had a lower incidence. Zofran seemed effective in lowering the NV severity as well. Therefore, recommending the systematic administration of antiemetic agents in patients receiving Oxycodone or Morphine could enhance patient satisfaction.
Worldwide, medical centers try to improve the health care system in its trilogy: care, health, and cost. Patient satisfaction is pointed out as an important clinical indicator for evaluating service quality. However, postoperative nausea and vomiting is still one of the most common and unpleasant side effects following surgeries under general anesthesia, with a mean overall incidence between 30-40% [1].
Perioperative opioid use remains one of the major participants in this issue. Opioids have been the mainstay of pain management for thousands of years, owing to their cost effectiveness in both the inpatient and outpatient settings. Aside from these attributes, opioids have significant side effects associated with nausea, vomiting, constipation, physical dependence, tolerance, and respiratory depression [2].
Morphine, the prototype opiate agent, traces its origin to single plant-the opium poppy. It has been used for centuries for recreation before it became widely used as a pain reliever, particularly in the 1800s. Due to its long analgesic duration of 4-5 hours, low cost, and extensive availability, it is often selected to control postoperative pain [3] preemptively.
Nevertheless, the disadvantages of morphine: slow onset of analgesia (20 min), unsuitability for hemodynamically unstable patients, and several other adverse effects have shed light on recent studies that adopt the use of alternative opioids or combination therapy to tackle this problem [4].
On the other hand, Oxycodone, a semi-synthetic opioid alkaloid derived from Thebaine, is a moderately potent opioid analgesic, developed in 1916 in Germany and has recently been used in Lebanon in an attempt to improve the existing opioids [5].
The recent interest in Oxycodone is based on its favorable pharmacokinetics and pharmacodynamics, especially in the central nervous system [6]. Moreover, relatively high enteral bioavailability allows an easy switch from one drug formulation to another during the course of pain management.
Oxycodone is highly effective and well tolerated in different types of surgical procedures and patient groups. One study conducted at New York University Langone Medical Center in the United States of America showed that the incidence of postoperative nausea and vomiting associated with Oxycodone is 19% [7], which is believed to surpass all the previous opioids, and mainly morphine.
Antiemetic agents were added to reduce one of the well-known side effects of opioids in postoperative settings. Zofran (Ondansetron) is a selective 5-HT3 receptor antagonist, approved by the FDA in 1991, triggers the area postrema in the brainstem [8], with primary effects in the gastrointestinal tract, reducing, therefore, nausea and vomiting within 30 min after IV or IM administration.
Our study aims to add to the previous research that aimed to find the best remedy for postoperative nausea and vomiting to improve patient satisfaction. Alongside pain, PONV is one of the most frequently encountered problems after surgical procedures, and its incidence could reach 30% [1]. While pain management is currently well managed, as well as morbidity and mortality from anesthetic agents, patients’ satisfaction is affected by their PONV experience [7]. Patients were sometimes willing to sacrifice by bearing pain, so they would not experience NV [4]. PONV could also affect patients’ bills by increasing their length of stay at the PACU. Therefore, recommendations to prevent and manage PONV are currently gaining interest, and research in this field is growing. Nausea and vomiting could be perceived as normal reactions of the body to any emetic stimulus. This includes a variety of stimuli such as food toxins and side effects of drug therapy. The stimulus is first detected by the abdominal vagal afferents, the area posterma, and the vestibular system. This leads to nausea, an unpleasant but nonpainful reaction, which prepares the body to learn either aversion or avoidance. Another reaction to this stimulus detection (that could also be present with it) is vomiting, a reflex reaction that helps to eject the toxins from the gastrointestinal tract. Studies showed that surgery duration, gender, age, smoking, BMI, pain level, and treatment agent were associated with a higher incidence of PONV [1]. A systematic review of the literature summarized evidence levels for all risk factors of PONV. The risk factors are mainly anesthesia dependents, such as volatile anesthetics and nitrous oxide administration, or concern the postoperative and intraoperative administration of opioids.
Prevention of PONV relies first on avoiding its risk factors where possible and preferring less emetic anesthetic agents and pain killers whenever possible. Zofran (Ondansetron) is a selective 5-HT3 receptor antagonist, approved by the FDA in 1991, triggers the area postrema in the brainstem [8], with primary effects in the gastrointestinal tract, reducing, therefore, nausea and vomiting within 30 min after IV administration. Moreover, its administration was known to reduce the incidence of PONV in adults [9] and pediatric population [10]. Besides, the Ondansetron effect with a 4mg dosage was found to be superior to 10 mg of metoclopramide in preventing PONV incidence and giving higher patient satisfaction scores in a systematic review of literature [11].
The incidence of nausea and vomiting immediately after the dose is 10% in Oxycodone group and 8.2% in Morphine group, without any statistically significant difference between the two treatment groups (Fisher’s Exact Test p-value = 0.636) (Figure 1).
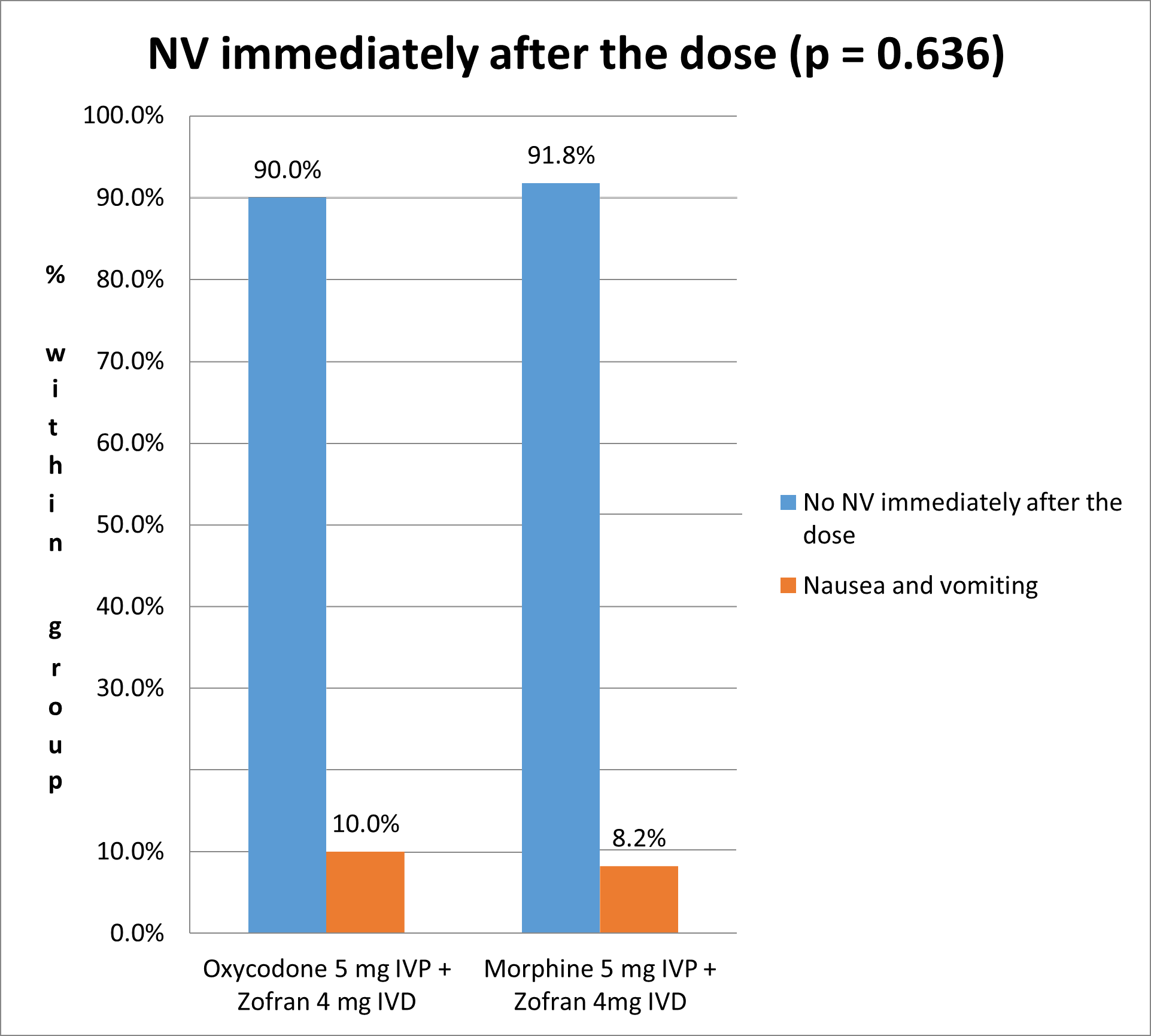
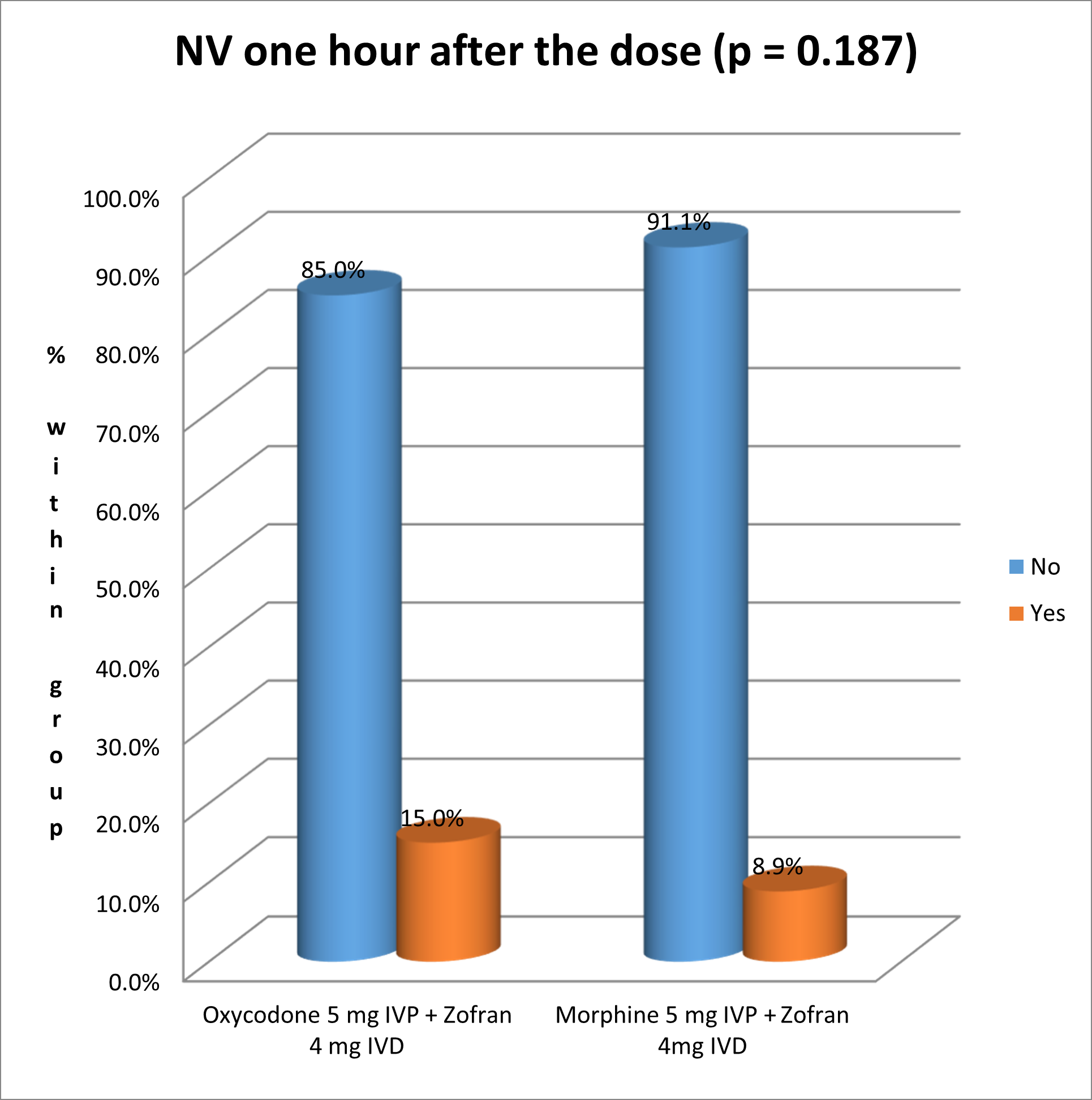
The following figure shows the incidence of nausea and vomiting one hour after the dose: 15% of Oxycodone patients experienced NV, versus 8.9 % in the group who received Morphine, without any statistically significant difference (Pearson’s chi square test p-value = 0.187) (Figure 2).
There was a statistically significant (Pearson’s chi square test p-value = 0.047 < 0.05) lower nausea and vomiting incidence in the Morphine group (2.5%) compared to the Oxycodone group (8.8% incidence) (Figure 3).
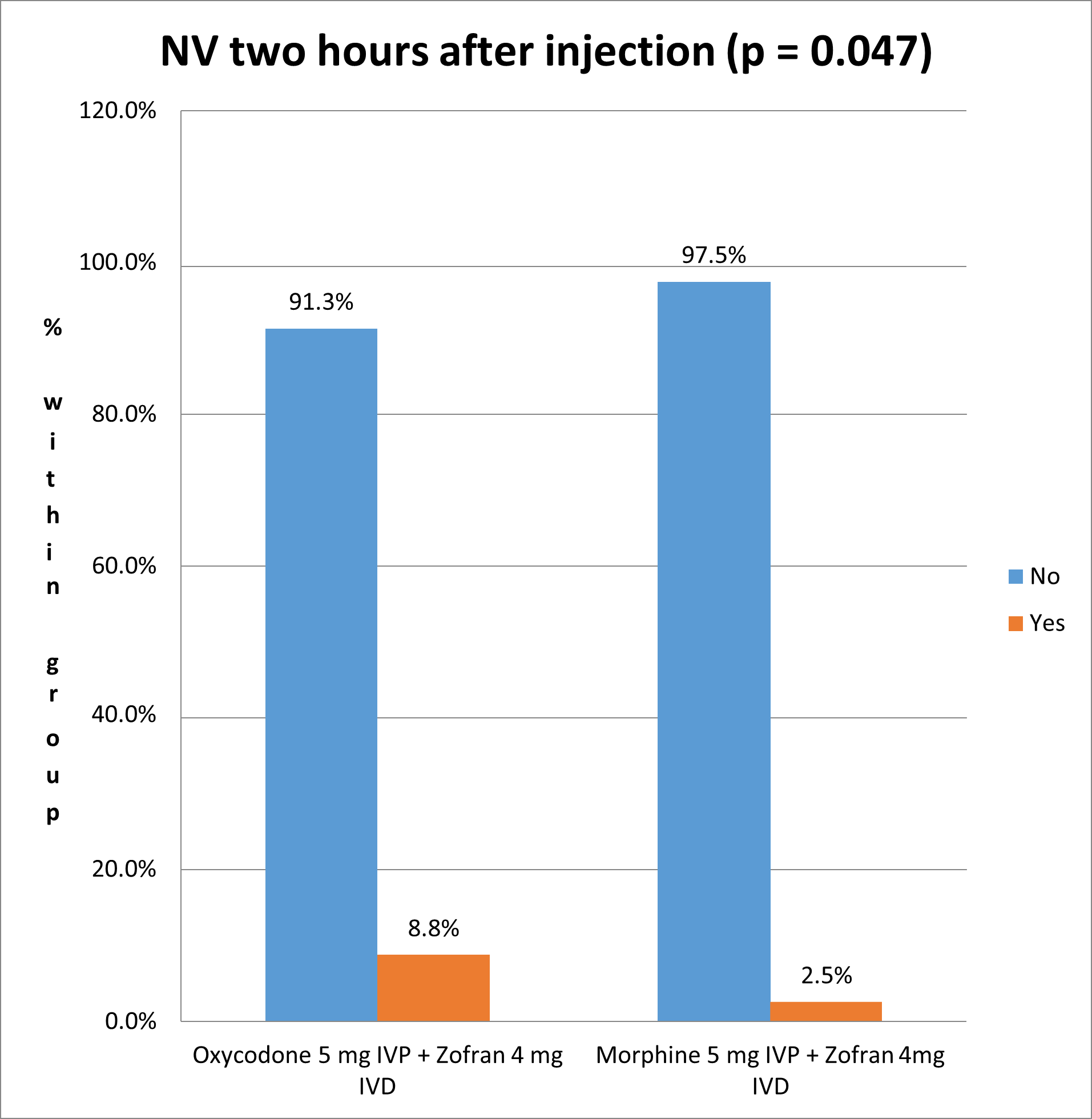
Our study showed a statistically significant (p-value = 0.047) lower nausea and vomiting incidence in the Morphine group (2.5%) compared to the Oxycodone group (8.8% incidence). These results match those found in 2013 by Pedersen and his colleagues, who concluded that Morphine had significantly less nausea than Oxycodone (p = 0.03) [13]. However, another study leaded by Lenz in 2009 found no difference between the two drugs in PONV incidence [14]. On the other hand, Oxycodone showed less secondary effects including sedation, when compared to Morphine and other opioids like Fentanyl and Sufentanyl [15]. Therefore, the controversy found in literature highlights the need for solid meta-analysis to get conclusive results about the safety and tolerance of Oxycodone and Morphine in postoperative pain management.
Moreover, our study aimed to assess the efficacy of Zofran for PONV prevention. Recent studies showed that the incidence of post-operative nausea and vomiting after oxycodone use post general anesthesia was 19% [7] vs 12% to 38% for morphine [12]. However, our results showed lower incidence rates of PONV: we found an 8.8% incidence in the Oxycodone group and a 2.5% incidence rate in the Morphine group. Therefore, the use of Zofran injection reduced by almost 2.16 times the incidence of NV in Oxycodone group, and by 4.8 to 15.2 times in the Morphine group. A 50% reduction of NV was considered clinically relevant by the researchers so the results obtained seemed way beyond expected and effectively very suitable for the patients.
Regarding PONV severity, our results showed that only 10% of Oxycodone group patients and 8.9% of Morphine group patients had moderate nausea and vomiting, and none of the groups’ patients had severe nausea and vomiting. To our knowledge, no other studies in the literature assessed the severity of NV in patients of our target population. However, since none of the patients reported severe nausea, then it seems that Zofran 4mg injection was effective in lowering the NV severity in the recruited patients (besides reducing its incidence).
Our study showed that neither surgery duration, nor gender, nor age, nor smoking, nor BMI, nor NRS pain level, nor treatment group were statistically associated with severity of nausea and vomiting. However, all the before mentioned were reported in other studies as risk factors of PONV [1,16]. The fact that none of these was detected as aggravating factors of PONV could be explained by the design of our study that included only a certain group of patients where all of them were with high pain levels, all of them receiving opioid injections, all being selected within a predefined age rank, which makes the homogeneity between the patients higher.
The study was conducted to find the best remedy to prevent PONV, one of the most common and unpleasant side effects following surgeries. Patients were sometimes willing to sacrifice by bearing pain so they would not experience NV [4]. Based on our results, measures to improve patient outcomes, public health, and health services should be taken, such as recommending the systematic administration of antiemetic agents to prevent or reduce the severity of PONV in patients receiving Oxycodone or Morphine.
Regarding analgesic effects, our results showed that Morphine and Oxycodone seemed to have similar analgesic effects. Our results also showed clinically relevant lower incidence rates of PONV in both Oxycodone and Morphine groups. Moreover, Zofran injection seemed effective in lowering the NV severity. Neither surgery duration, gender, age, smoking, BMI, nor NRS pain level, nor treatment group was statistically associated with the severity of nausea and vomiting.
The study was conducted to find the best remedy to prevent PONV, one of the most common and unpleasant side effects following surgeries. Based on our results, recommending the systematic administration of antiemetic agents to prevent or reduce the severity of PONV in patients receiving Oxycodone or Morphine could enhance patients’ satisfaction. Future studies could focus on including all the factors affecting PONV incidence and evaluate all their side effects to accurately estimate the efficacy and safety of Zofran as an antiemetic.
